Flovent HFA
Brand names: Arnuity Ellipta, Flovent Diskus, Flovent HFA
Drug class: Inhaled corticosteroids
What is Flovent HFA?
Flovent HFA is a prescription inhaled corticosteroid (ICS) medicine used for the long-term treatment of asthma in people aged 4 years and older.
ICS medicines such as fluticasone propionate help to decrease inflammation in the lungs. Inflammation in the lungs can lead to breathing problems. Flovent HFA is not used to relieve sudden breathing problems and will not replace a rescue inhaler. It is not known if Flovent HFA is safe and effective in children younger than 4 years of age.
Who should not use Flovent HFA?
Do not use Flovent HFA:
- to relieve sudden breathing problems.
- as a rescue inhaler.
- if you are allergic to fluticasone propionate or any of the ingredients in Flovent HFA. See the end of this guide for a complete list of ingredients in Flovent HFA.
Description
Flovent HFA is a pressurized metered dose inhaler for oral inhalation. The active component of FLOVENT HFA 44 mcg, FLOVENT HFA 110 mcg, and FLOVENT HFA 220 mcg is fluticasone propionate, a corticosteroid having the chemical name S-(fluoromethyl) 6α,9-difluoro-11β,17-dihydroxy-16α-methyl-3-oxoandrosta-1,4-diene-17β-carbothioate, 17-propionate and the following chemical structure:
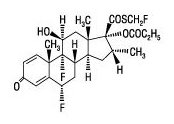
Fluticasone propionate is a white powder with a molecular weight of 500.6, and the empirical formula is C25H31F3O5S. It is practically insoluble in water, freely soluble in dimethyl sulfoxide and dimethylformamide, and slightly soluble in methanol and 95% ethanol.
Flovent HFA is a dark orange plastic inhaler with a peach cap containing a pressurized metered-dose aerosol canister fitted with a counter. Each canister contains a microcrystalline suspension of micronized fluticasone propionate in propellant HFA-134a (1,1,1,2-tetrafluoroethane). It contains no other excipients.
After priming, each actuation of the inhaler delivers 50, 125, or 250 mcg of fluticasone propionate in 60 mg of suspension (for the 44-mcg product) or in 75 mg of suspension (for the 110- and 220-mcg products) from the valve. Each actuation delivers 44, 110, or 220 mcg of fluticasone propionate from the actuator. The actual amount of drug delivered to the lung will depend on patient factors, such as the coordination between the actuation of the inhaler and inspiration through the delivery system.
Prime FLOVENT HFA before using for the first time by releasing 4 sprays into the air away from the face, shaking well for 5 seconds before each spray. In cases where the inhaler has not been used for more than 7 days or when it has been dropped, prime the inhaler again by shaking well for 5 seconds and releasing 1 spray into the air away from the face. Avoid spraying in eyes.
Mechanism of Action
Fluticasone propionate is a synthetic trifluorinated corticosteroid with anti-inflammatory activity. Fluticasone propionate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor that is 18 times that of dexamethasone, almost twice that of beclomethasone‑17‑monopropionate (BMP), the active metabolite of beclomethasone dipropionate, and over 3 times that of budesonide. Data from the McKenzie vasoconstrictor assay in man are consistent with these results. The clinical significance of these findings is unknown.
Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of actions on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, cytokines) involved in inflammation. These anti-inflammatory actions of corticosteroids contribute to their efficacy in asthma.
Though effective for the treatment of asthma, corticosteroids do not affect asthma symptoms immediately. Individual patients will experience a variable time to onset and degree of symptom relief. Maximum benefit may not be achieved for 1 to 2 weeks or longer after starting treatment. When corticosteroids are discontinued, asthma stability may persist for several days or longer.
Trials in subjects with asthma have shown a favorable ratio between topical anti-inflammatory activity and systemic corticosteroid effects with recommended doses of orally inhaled fluticasone propionate. This is explained by a combination of a relatively high local anti-inflammatory effect, negligible oral systemic bioavailability (<1%), and the minimal pharmacological activity of the only metabolite detected in man.
What should I tell my healthcare provider before using Flovent HFA?
Before using Flovent HFA, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems.
- have weak bones (osteoporosis).
- have an immune system problem.
- have or have had eye problems such as glaucoma, increased pressure in your eye, cataracts, or other changes in vision.
- have any type of viral, bacterial, or fungal infection.
- are exposed to chickenpox or measles.
- are pregnant or plan to become pregnant. It is not known if Flovent HFA may harm your unborn baby.
- are breastfeeding. It is not known if the medicine in Flovent HFA passes into your milk and if it can harm your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Flovent HFA and certain other medicines may interact with each other. This may cause serious side effects. Especially tell your healthcare provider if you take antifungal or anti-HIV medicines.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Flovent HFA?
Read the step-by-step instructions for using Flovent HFA at the end of this Patient Information.
- Do not use Flovent HFA unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- Children should use Flovent HFA with an adult’s help, as instructed by the child’s healthcare provider.
- Flovent HFA comes in 3 different strengths. Your healthcare provider prescribed the strength that is best for you.
- Use Flovent HFA exactly as your healthcare provider tells you to use it. Do not use Flovent HFA more often than prescribed.
- It may take 1 to 2 weeks or longer after you start Flovent HFA for your asthma symptoms to get better. You must use Flovent HFA regularly.
- Do not stop using Flovent HFA, even if you are feeling better, unless your healthcare provider tells you to.
- If you miss a dose of Flovent HFA, just skip that dose. Take your next dose at your usual time. Do not take 2 doses at 1 time.
- Flovent HFA does not relieve sudden breathing problems. Always have a rescue inhaler with you to treat sudden symptoms. If you do not have a rescue inhaler, call your healthcare provider to have one prescribed for you.
- Rinse your mouth with water without swallowing after each dose of Flovent HFA. This will help lessen the chance of getting a yeast infection (thrush) in your mouth and throat.
- Call your healthcare provider or get medical care right away if:
- your breathing problems get worse.
- you need to use your rescue inhaler more often than usual.
- your rescue inhaler does not work as well to relieve your symptoms.
- you need to use 4 or more inhalations of your rescue inhaler in 24 hours for 2 or more days in a row.
- you use 1 whole canister of your rescue inhaler in 8 weeks.
- your peak flow meter results decrease. Your healthcare provider will tell you the numbers that are right for you.
What are the possible side effects of Flovent HFA?
Flovent HFA can cause serious side effects, including:
- fungal infection in your mouth or throat (thrush). Rinse your mouth with water without swallowing after using Flovent HFA to help reduce your chance of getting thrush.
- weakened immune system and increased chance of getting infections (immunosuppression).
- reduced adrenal function (adrenal insufficiency). Adrenal insufficiency is a condition where the adrenal glands do not make enough steroid hormones. This can happen when you stop taking oral corticosteroid medicines (such as prednisone) and start taking a medicine containing an inhaled steroid (such as Flovent HFA). During this transition period, when your body is under stress such as from fever, trauma (such as a car accident), infection, or surgery, adrenal insufficiency can get worse and may cause death. Symptoms of adrenal insufficiency include:
- serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction:
- rash
- swelling of your face, mouth, and tongue
- hives
- breathing problems
- bone thinning or weakness (osteoporosis).
- slowed growth in children. Your child’s growth should be checked regularly by the healthcare provider while using Flovent HFA.
- eye problems including glaucoma, increased pressure in your eye, cataracts, or other changes in vision. You should have regular eye exams while using Flovent HFA.
- increased wheezing (bronchospasm). Increased wheezing can happen right away after using Flovent HFA. Always have a rescue inhaler with you to treat sudden wheezing.
Common side effects of Flovent HFA include:
- upper respiratory tract infection
- diarrhea
- cough
- throat irritation
- difficulty speaking
- persistent cough
- headache
- sinus irritation
These are not all the possible side effects of Flovent HFA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Flovent HFA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Flovent HFA for a condition for which it was not prescribed. Do not give Flovent HFA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Flovent HFA that was written for health professionals.
How should I store Flovent HFA?
- Store Flovent HFA at room temperature between 68°F and 77°F (20°C and 25°C) with the mouthpiece down.
- The contents of your Flovent HFA inhaler are under pressure. Do not puncture. Do not use or store near heat or open flame. Temperatures above 120°F may cause the canister to burst.
- Do not throw into fire or an incinerator.
- Safely throw away Flovent HFA in the trash when the counter reads 000.
Keep Flovent HFA and all medicines out of the reach of children.
What are the ingredients in Flovent HFA?
Active ingredient: fluticasone propionate
Inactive ingredient: propellant HFA-134a
For more information about Flovent HFA, call 1-888-825-5249.
Instructions for use for Flovent HFA
Flovent (FLO vent) HFA
(fluticasone propionate inhalation aerosol)
for oral inhalation use
Your Flovent HFA inhaler
- The metal canister holds the medicine. See Figure A.
- The metal canister has a counter to show how many sprays of medicine you have left. The number shows through a window in the back of the dark orange plastic actuator. See Figure A.
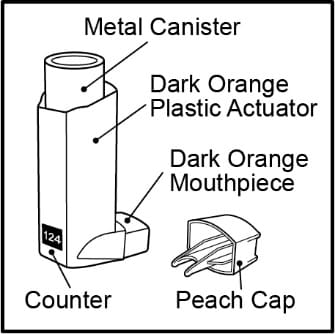
Figure A
- The counter starts at 124. The number will count down by 1 each time you spray the inhaler. The counter will stop counting at 000.
- Do not try to change the numbers or take the counter off the metal canister. The counter cannot be reset, and it is permanently attached to the metal canister.
- The dark orange plastic actuator sprays the medicine from the metal canister. The plastic actuator has a peach protective cap that covers the mouthpiece. See Figure A. Keep the protective cap on the mouthpiece when the metal canister is not in use.
- Do not use the plastic actuator with a canister of medicine from any other inhaler.
- Do not use a Flovent HFA metal canister with an actuator from any other inhaler.
Before using your Flovent HFA inhaler
- The inhaler should be at room temperature before you use it.
- If a child needs help using the inhaler, an adult should help the child use the inhaler with or without a valved holding chamber, which may also be attached to a mask. The adult should follow the instructions that came with the valved holding chamber. An adult should watch a child use the inhaler to be sure it is used correctly.
Priming your Flovent HFA inhaler
Before you use Flovent HFA for the first time, you must prime the inhaler so that you will get the right amount of medicine when you use it.
- To take the cap off the mouthpiece, squeeze the sides of the cap and pull it straight out. See Figure B.
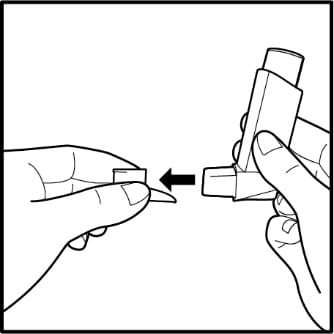
Figure B
- Shake the inhaler well for 5 seconds.
- Spray the inhaler 1 time into the air away from your face. Avoid spraying in eyes. See Figure C.
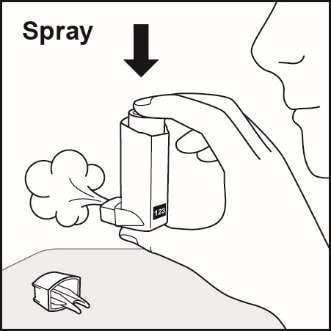
Figure C
- Shake and spray the inhaler like this 3 more times to finish priming it. The counter should now read 120. See Figure D.
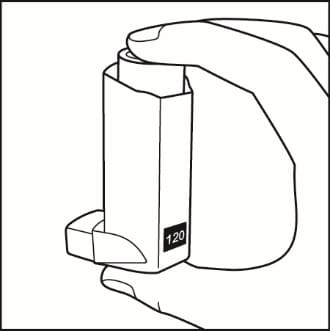
Figure D
- You must prime your inhaler again if you have not used it in more than 7 days or if you have dropped it. To take the cap off the mouthpiece, squeeze the sides of the cap and pull it straight out. Shake the inhaler well for 5 seconds. Then spray it 1 time into the air away from your face.
How to use your Flovent HFA inhaler
Follow these steps every time you use Flovent HFA.
Step 1. Make sure the metal canister fits firmly in the plastic actuator. The counter should show through the window in the plastic actuator.
- To take the cap off the mouthpiece, squeeze the sides of the cap and pull it straight out.
- Look inside the mouthpiece for foreign objects and take out any you see.
Step 2. Hold the inhaler with the mouthpiece down and shake it well for 5 seconds . See Figure E.
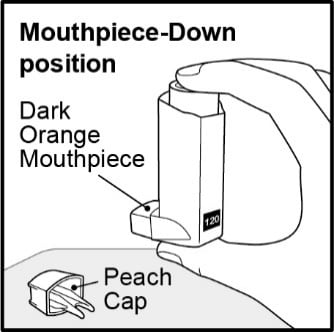
Figure E
Step 3. Breathe out through your mouth and push as much air from your lungs as you can. See Figure F.
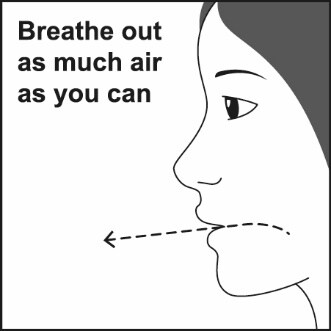
Figure F
Step 4. Put the mouthpiece in your mouth and close your lips around it. Push the top of the metal canister firmly all the way down while you breathe in deeply and slowly through your mouth. See Figure G.
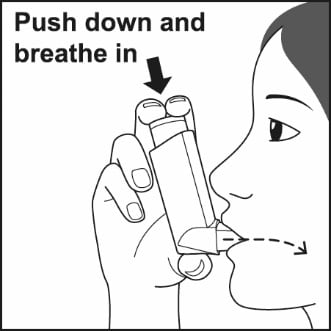
Figure G
Step 5. After the spray comes out, take your finger off the metal canister. After you have breathed in all the way, take the inhaler out of your mouth and close your mouth.
Step 6. Hold your breath for about 10 seconds, or for as long as is comfortable. Breathe out slowly as long as you can.
Wait about 30 seconds and shake the inhaler well for 5 seconds. Repeat Step 2 through Step 6.
Step 7. Rinse your mouth with water after breathing in the medicine. Spit out the water. Do not swallow it. See Figure H.
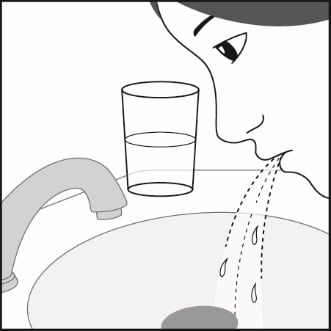
Figure H
Step 8. Put the cap back on the mouthpiece after you finish using the inhaler. Make sure it snaps firmly into place.
Cleaning your Flovent HFA inhaler
Clean your inhaler at least 1 time each week after your evening dose. You may not see any medicine build-up on the inhaler, but it is important to keep it clean so medicine build-up will not block the spray. See Figure I.
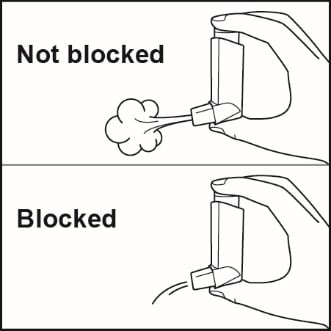
Figure I
Step 9. Take the cap off the mouthpiece by squeezing the sides of the cap and pulling it straight out. Do not take the metal canister out of the plastic actuator.
Step 10. Use a clean cotton swab dampened with water to clean the small circular opening where the medicine sprays out of the metal canister. Gently twist the swab in a circular motion to take off any medicine. See Figure J. Repeat with a new swab dampened with water to take off any medicine still at the opening.
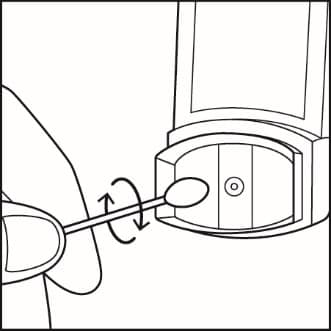
Figure J
Step 11. Wipe the inside of the mouthpiece with a clean tissue dampened with water. Let the plastic actuator air-dry overnight.
Step 12. Put the cap back on the mouthpiece after the plastic actuator has dried.
Replacing your Flovent HFA inhaler
- When the counter reads 020, you should refill your prescription or ask your healthcare provider if you need another prescription for Flovent HFA.
- When the counter reads 000, throw the inhaler away. You should not keep using the inhaler when the counter reads 000 because you may not receive the right amount of medicine.
- Do not use the inhaler after the expiration date, which is on the packaging it comes in.
For correct use of your Flovent HFA inhaler, remember:
- The metal canister should always fit firmly in the plastic actuator.
- Shake the inhaler well for 5 seconds before each spray.
- Breathe in deeply and slowly to make sure you get all the medicine.
- Hold your breath for about 10 seconds after breathing in the medicine. Then breathe out fully.
- After each dose, rinse your mouth with water and spit it out. Do not swallow the water.
- Do not take the inhaler apart.
- Always keep the protective cap on the mouthpiece when your inhaler is not in use.
- Always store your inhaler with the mouthpiece pointing down.
- Clean your inhaler at least 1 time each week.
For more information about Flovent HFA or how to use your inhaler, call 1-888-825-5249.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0173-0719-20
- Flovent HFA
- (fluticasone propionate inhalation aerosol)
- 110 mcg
- For oral inhalation with FLOVENT HFA actuator only.
- Attention Pharmacist: Detach Patient Information leaflet from package insert and dispense with inhaler.
- See prescribing information for dosage information.
- Rx only
- Net Wt. 12 g
- 120 Metered Actuations
- gsk
- ©2021 GSK group of companies or its licensor.
- Made in Singapore
- 62000000062557 Rev. 5/21
PRINCIPAL DISPLAY PANEL
- NDC 0173-0720-20
- Flovent HFA
- (fluticasone propionate inhalation aerosol)
- 220 mcg
- For oral inhalation with FLOVENT HFA actuator only.
- Attention Pharmacist: Detach Patient Information leaflet from package insert and dispense with inhaler.
- See prescribing information for dosage information.
- Rx only
- Net Wt. 12 g
- 120 Metered Actuations
- gsk
- ©2021 GSK group of companies or its licensor.
- Made in Singapore
- 62000000062558 Rev. 5/21
SRC: NLM .
