Flolan
Generic name: epoprostenol
Brand names: Flolan, Veletri
Drug class: Agents for pulmonary hypertension
Medically reviewed by A Ras MD.
What is Flolan?
Flolan is a prescription medicine used to treat people with certain types of pulmonary arterial hypertension (PAH), which is high blood pressure in the arteries of the lungs. Flolan can improve your ability to be physically active.
It is not known if Flolan is safe and effective in children.
Description
FLOLAN (epoprostenol sodium) for injection is sterile sodium salt that is a white or off-white powder formulated for intravenous (IV) administration. Each vial of FLOLAN contains epoprostenol sodium equivalent to either 0.5 mg (500,000 ng) or 1.5 mg (1,500,000 ng) epoprostenol, 3.76 mg glycine, 50 mg mannitol, and 2.93 mg sodium chloride. Sodium hydroxide may have been added to adjust pH.
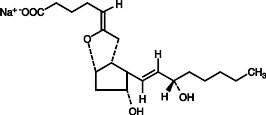
Epoprostenol (PGI2, PGX, prostacyclin), a metabolite of arachidonic acid, is a naturally occurring prostaglandin with potent vasodilatory activity and inhibitory activity of platelet aggregation. The chemical name of epoprostenol is (5Z,9α,11α,13E,15S)-6,9-epoxy-11,15-dihydroxyprosta-5,13-dien-1-oic acid. Epoprostenol sodium has a molecular weight of 374.45 and a molecular formula of C20H31NaO5. The structural formula is:
FLOLAN must be reconstituted with pH 12 STERILE DILUENT for FLOLAN.
pH 12 STERILE DILUENT for FLOLAN is supplied in plastic vials each containing 50 mL of 94 mg glycine, 73.3 mg sodium chloride, sodium hydroxide (added to adjust the pH to 11.7 to 12.3), and Water for Injection. The stability of reconstituted solutions of FLOLAN is pH-dependent and is greater at higher pH.
Mechanism of Action
Epoprostenol has 2 major pharmacological actions: (1) direct vasodilation of pulmonary and systemic arterial vascular beds and (2) inhibition of platelet aggregation.
Who should not use Flolan?
Do not use Flolan if you:
- have certain types of heart failure. Talk to your healthcare provider before using Flolan if you have heart failure.
- are allergic to Flolan or any of the ingredients in Flolan. See the end of this leaflet for a complete list of ingredients in Flolan.
What should I tell my healthcare provider before using Flolan?
Before you use Flolan, tell your healthcare provider about all of your medical conditions, including if you:
- are allergic to any medicine.
- are pregnant or plan to become pregnant. It is not known if Flolan will harm your unborn baby. You and your healthcare provider should decide if you will use Flolan.
- are breastfeeding or plan to breastfeed. It is not known if Flolan passes into your breast milk. You and your healthcare provider should decide if you will take Flolan or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- a “water pill” (diuretic)
- a medicine for high blood pressure (hypertension)
- a blood thinner medicine (antiplatelet or anticoagulant medicine)
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Flolan?
- Flolan should only be given by infusion through a catheter placed in a vein (intravenous infusion) using an infusion pump.
- Your first treatment will be given to you by your healthcare provider or nurse. This is so your healthcare provider can monitor you and find the best dose for you.
- If your healthcare provider decides that you or your caregiver can give infusions of Flolan at home, you or your caregiver will receive training on the right way to mix and infuse Flolan. Do not try to infuse Flolan until you have been shown the right way to infuse Flolan by your healthcare provider.
- Treatment will be needed for a long period of time, possibly years. You must be able to accept and care for a catheter and infusion pump in order to be treated with Flolan.
- Use Flolan exactly as your healthcare provider tells you to.
- Do not change your dose or stop your infusion without talking to your healthcare provider. Stopping Flolan suddenly can cause serious side effects.
- You should have a backup infusion pump and extra supplies needed for your infusion of Flolan.
- Follow your healthcare provider’s instructions for taking blood thinner medicines, if prescribed for you.
- Before you use Flolan, you must mix (reconstitute) Flolan powder with a diluent. There are 2 different types of diluents:
- Sterile Diluent for Flolan (comes in a glass bottle)
- pH 12 Sterile Diluent for Flolan (comes in a plastic bottle)
- Do not mix Flolan with any other diluent. You must use Sterile Diluent for Flolan or pH 12 Sterile Diluent for Flolan.
- Flolan prepared with pH 12 Sterile Diluent for Flolan must not be used with any preparation or administration materials containing polyethylene terephthalate (PET) or polyethylene terephthalate glycol (PETG). Only use materials provided by a healthcare provider or pharmacist.See “How should I store Flolan?” for more information about how to use and store Flolan the right way.
- A mixed solution of Flolan is clear and colorless. Do not use Flolan if the mixed solution looks discolored or cloudy, or if the solution has flakes or particles in it.
Using more than the prescribed dose of Flolan can lead to death. If you use more than the prescribed dose of Flolan, call your healthcare provider or go to the nearest emergency room right away.
What are the possible side effects of Flolan?
Flolan can cause serious side effects, including:
- Fluid in your lungs (pulmonary edema). If you develop pulmonary edema after starting Flolan, your healthcare provider will stop your treatment and you should not receive Flolan again.
- Worsening symptoms of pulmonary arterial hypertension (PAH) with a sudden decrease in the dose of Flolan. Do not change your dose of Flolan or stop your infusion without talking to your healthcare provider. If you suddenly stop or decrease your dose of Flolan you may develop worsening symptoms of your PAH, including shortness of breath, dizziness, weakness, or loss of strength.
- Widening of your blood vessels (vasodilation). Vasodilation reactions can happen after you start Flolan. These reactions are common and may cause low blood pressure (hypotension), flushing, nausea, vomiting, dizziness, and headache. Your healthcare provider should check your blood pressure regularly during treatment with Flolan, especially when you start Flolan and after your dose is changed.
- Increased risk for bleeding. Flolan affects how well your blood clots, so your risk for bleeding is increased. This is especially true if you have other risk factors for bleeding. Tell your healthcare provider if you develop any unusual bruising or bleeding.
The most common side effects of Flolan include:
- dizziness
- jaw pain
- headache
- muscle or bone pain
- nausea or vomiting
These are not all the possible side effects of Flolan. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Flolan
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Flolan for a condition for which it was not prescribed. Do not give Flolan to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about Flolan. You can ask your healthcare provider or pharmacist for information about Flolan that is written for health professionals.
How should I store Flolan?
- Store Flolan powder at room temperature between 59°F to 77°F (15°C to 25°C).
- Protect Flolan powder from light. Keep unopened vial of Flolan in the carton until you are ready to mix.
- Store the Sterile Diluent for Flolan and the pH 12 Sterile Diluent for Flolan at room temperature, 59°F to 77°F (15°C to 25°C). Do not freeze.
- Vials of Sterile Diluent for Flolan, and pH 12 Sterile Diluent for Flolan are for one-time use only. Throw away any unused diluent.
- Throw away any vials of Flolan powder, Sterile Diluent for Flolan, and pH 12 Sterile Diluent for Flolan that are out of date or that you no longer need.
How to store mixed solutions of Flolan:
- Once Flolan and the diluent are mixed together, you may use right away or store in the refrigerator. Refrigerate at 36°F to 46°F (2°C to 8°C).
- Protect the mixed solution of Flolan from light until you are ready to use it.
- Do not freeze mixed solutions. Throw away any mixed solution that has been frozen.
- If you are using Sterile Diluent for Flolan (comes in a glass bottle) for mixing:
- If the mixed solution will be used at room temperature:
- Use the mixed solution over a period of no longer than 8 hours after mixing if not stored in the refrigerator.
- If the mixed solution has been stored in the refrigerator, infuse it over a period of no longer than 8 hours after removing it from the refrigerator.
- You may store the mixed solution for up to 40 hours in the refrigerator.
- Throw away any mixed solution if it has been refrigerated for more than 40 hours.
- If the mixed solution will be used with a cold pouch:
- You may store the mixed solution in the refrigerator for up to 24 hours.
- Take the mixed solution out of the refrigerator and use it with the cold pouch over a period of no longer than 24 hours. Change the cold pouch every 12 hours.
- The mixed solution may be kept either in the refrigerator or in the cold pouch, or a combination of the two, for no more than 48 hours. After 48 hours, throw away any mixed solution.
- If you are using pH 12 Sterile Diluent for Flolan (comes in a plastic bottle) for mixing:
- Freshly prepared mixed solutions may be stored in the refrigerator for up to 8 days.
- Mixed solutions (freshly prepared or taken out of the refrigerator) are stable for up to 3 days at 77°F (25°C), up to 2 days at 86°F (30°C), up to 1 day at 95°F (35°C) or up to 12 hours at 104°F (40°C).
- Flolan mixed with pH 12 Sterile Diluent for Flolan does not require use with a cold pouch.
- Throw away any mixed solution if it has been refrigerated for more than 8 days.
Keep Flolan and all medicines out of the reach of children.
What are the ingredients in Flolan?
Active ingredient: epoprostenol sodium.
Inactive ingredients: glycine, mannitol, sodium chloride. Sodium hydroxide may have been added.
The Sterile Diluent for Flolan and the pH 12 Sterile Diluent for Flolan contain: glycine, sodium chloride, sodium hydroxide, and Water for Injection.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0173-0519-00
- FLOLAN®
- (epoprostenol sodium)
- for Injection
- 1.5 mg/vial (1,500,000 ng/vial)
- Must be reconstituted. For intravenous infusion after dilution.
- Note: Use only STERILE DILUENT for FLOLAN or pH 12 STERILE DILUENT for FLOLAN for reconstitution and dilution.
- Rx only
- Single-Use Vial
- Each vial contains epoprostenol sodium equivalent to 1.5 mg (1,500,000 ng) epoprostenol, 3.76 mg glycine, 2.93 mg sodium chloride, and 50 mg mannitol. Sodium hydroxide may have been added to adjust pH.
- Usual Dose: See prescribing information.
- Store at 15o and 25o C (59o to 77oF).
- Do not accept if plastic overseal is missing or not securely fitted.
- GlaxoSmithKline
- RTP, NC 27709
- Rev. 4/16
- 10000000140667
PRINCIPAL DISPLAY PANEL
- NDC 0173-0857-02
- pH 12 Sterile Diluent for FLOLAN®
- Use only with FLOLAN® (epoprostenol sodium) for Injection
- Single-Use Vial.
- Discard Unused Portion
- 50 Ml
- Rx only
- For reconstitution information see package insert for FLOLAN® (epoprostenol sodium) for Injection.
- Store at 15° to 25°C (59° to 77°F).
- DO NOT FREEZE.
- Do not accept if plastic overseal is missing or not securely fitted.
- GlaxoSmithKline, RTP, NC 27709
- Made in England
- 10000000133560 Rev. 3/15

SRC: NLM .
