Fensolvi
Generic name: leuprolide acetate
Dosage form: injectable suspension, for subcutaneous use
Drug classes: Gonadotropin releasing hormones, Hormones / antineoplastics
Medically reviewed by A Ras MD.
What is Fensolvi?
Fensolvi is a prescription gonadotropin releasing hormone (GnRH) medicine used for the treatment of children with central precocious puberty (CPP). It is not known if Fensolvi is safe and effective in children younger than 2 years of age.
Description
FENSOLVI for injectable suspension is a sterile polymeric matrix formulation of leuprolide acetate, a GnRH agonist, for subcutaneous use. It is designed to deliver leuprolide acetate at a controlled rate over a six-month therapeutic period.
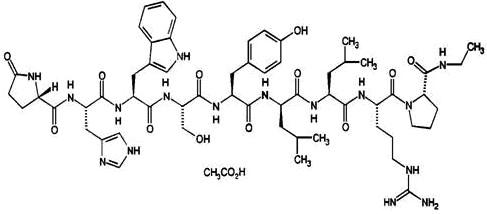
Leuprolide acetate is a synthetic nonapeptide analog of naturally occurring gonadotropin releasing hormone. The analog possesses greater potency than the natural hormone. The chemical name is 5-oxo-L-prolyl-L-histidyl-L-tryptophyl-L-seryl-L-tyrosyl-D-leucyl-L-leucyl-L-arginyl-N-ethyl-L-prolinamide acetate (salt) with the following structural formula:
FENSOLVI is prefilled and supplied in two separate, sterile syringes whose contents are mixed immediately prior to administration. The two syringes are joined and the single dose product is mixed until it is homogenous. FENSOLVI is administered subcutaneously, where it forms a solid drug delivery depot.
One syringe contains the ATRIGEL Delivery System and the other contains the leuprolide acetate. ATRIGEL is a polymeric (non-gelatin containing) delivery system consisting of a biodegradable poly(DL-lactide-co-glycolide) (PLG) polymer formulation dissolved in the biocompatible solvent, N-methyl-2-pyrrolidone (NMP).
Refer to Table 3 for the delivery system composition and reconstituted product formulation for FENSOLVI product.
Table 3: FENSOLVI Delivery System Composition and Reconstituted Product Formulation
| ATRIGEL Delivery System Syringe | Polymer | PLG |
| Polymer description | Copolymer with hexanediol | |
| Polymer DL-lactide to glycolide molar ratio | 85:15 | |
|
Reconstituted Product |
Polymer delivered |
165 mg |
| NMP delivered | 165 mg | |
| Leuprolide acetate delivered | 45 mg | |
| Approximate leuprolide free base equivalent | 42 mg | |
| Approximate administered formulation weight | 375 mg | |
| Approximate injection volume | 0.375 mL |
Mechanism of Action
Leuprolide acetate, a GnRH agonist, acts as a potent inhibitor of gonadotropin secretion (LH and follicle stimulating hormone (FSH)) when given continuously in therapeutic doses. Following an initial stimulation of GnRH receptors, chronic administration of leuprolide acetate results in downregulation of GnRH receptors, reduction in release of LH, FSH and consequent suppression of ovarian and testicular production of estradiol and testosterone respectively. This inhibitory effect is reversible upon discontinuation of drug therapy.
What is the most important information I should know about Fensolvi?
Fensolvi may cause serious side effects, including:
- In the first few weeks after your child receives their first Fensolvi injection, Fensolvi can cause an increase in some hormones. During this time, you may notice more signs of puberty in your child including vaginal bleeding. Call your child’s healthcare provider if signs of puberty continue after 2 months of receiving Fensolvi.
- Some people taking gonadotropin releasing hormone (GnRH) agonists like Fensolvi have had new or worsened mental (psychiatric) problems. Mental (psychiatric) problems may include emotional symptoms such as:
- crying
- irritability
- restlessness (impatience)
- anger
- acting aggressively
Call your child’s healthcare provider right away if your child has any new or worsening emotional symptoms while taking Fensolvi.
- Some people taking GnRH agonists like Fensolvi have had seizures. The risk of seizures may be higher in people who:
- have a history of seizures
- have a history of epilepsy
- have a history of brain tumors or brain vessel (cerebrovascular) problems
- are taking a medicine that has been associated with seizures such as bupropion or selective serotonin reuptake inhibitors (SSRIs)
Seizures have also happened in people who have not had any of these problems.
Call your child’s healthcare provider right away if your child has a seizure while taking Fensolvi.
Who should not use Fensolvi?
Fensolvi should not be received if your child is:
- allergic to GnRH, GnRH agonist medicines, or any ingredients in Fensolvi. See the end of this Medication Guide for a complete list of ingredients in Fensolvi.
Call your child’s healthcare provider or get emergency medical help right away if your child gets any of the following symptoms of a serious allergic reaction: - pregnant or becomes pregnant. Fensolvi can cause birth defects or loss of the baby. If your child becomes pregnant, call your healthcare provider.
What should I tell my healthcare provider before using Fensolvi?
Before your child receives Fensolvi, tell your child’s healthcare provider about all of your child’s medical conditions, including if they:
- have a history of mental (psychiatric) problems.
- have a history of seizures.
- have a history of epilepsy.
- have a history of brain vessel (cerebrovascular) problems.
- have a history of brain or spinal cord (central nervous system) problems or tumors.
- are breastfeeding or plan to breastfeed. It is not known if Fensolvi passes into breastmilk.
Tell your healthcare providerabout all the medicines your child takes, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Fensolvi?
- Fensolvi will be given to your child by a healthcare provider.
- Fensolvi is injected under the skin (subcutaneous) every 6 months.
- Keep all scheduled visits to the healthcare provider. If you miss a scheduled dose, your child may start having signs of puberty again. The healthcare provider will do regular exams and blood tests to check for signs of puberty.
What are the possible side effects of Fensolvi?
Fensolvi may cause serious side effects. See “What is the most important information I should know about Fensolvi?”
The most common side effects of Fensolvi include:
- injection site pain
- abdominal pain
- sudden shortness of breath or wheezing (bronchospasm)
- nasal congestion, sore throat, and runny nose (nasopharyngitis)
- nausea
- productive cough
- fever (pyrexia)
- constipation
- sudden strong feelings of heat and sweating (hot flush)
- headache
- vomiting
- cough
- upper respiratory tract infection
These are not all the possible side effects of Fensolvi. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Fensolvi
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide.
You can ask your pharmacist or healthcare provider for information about Fensolvi that is written for health professionals.
How should I store Fensolvi?
Store refrigerated at 2 – 8°C (35.6 – 46.4°F).
Once outside the refrigerator, this product may be stored in its original packaging at room temperature 15 – 30°C (59 – 86°F) for up to eight weeks prior to reconstitution and administration.
What are the ingredients in Fensolvi?
Active ingredient: leuprolide acetate.
Inactive ingredients: poly (DL-lactide-co-glycolide) and N-methyl-2-pyrrolidone.
Label
PRINCIPAL DISPLAY PANEL
SRC: NLM .
