Felbatol
Generic name: felbamate
Drug class: Carbamate anticonvulsants
Medically reviewed by A Ras MD.
What is Felbatol?
Felbatol is a prescription medicine used when other treatments have failed in adults alone or with other medicines to treat partial seizures with and without generalization children with other medicines to treat seizures associated with Lennox-Gastaut syndrome
Description
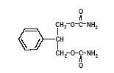
Felbatol® (felbamate) is an antiepileptic available as 400 mg and 600 mg tablets and as a 600 mg/5 mL suspension for oral administration. Its chemical name is 2-phenyl-1,3-propanediol dicarbamate.
Felbamate is a white to off-white crystalline powder with a characteristic odor. It is very slightly soluble in water, slightly soluble in ethanol, sparingly soluble in methanol, and freely soluble in dimethyl sulfoxide. The molecular weight is 238.24; felbamate’s molecular formula is C 11 H 14 N 2 O 4 ; its structural formula is:
The inactive ingredients for Felbatol® (felbamate) Tablets 400 mg and 600 mg are starch, microcrystalline cellulose, croscarmellose sodium, lactose, magnesium stearate, FD&C Yellow No. 6, D&C Yellow No. 10, and FD&C Red No. 40 (600 mg tablets only). The inactive ingredients for Felbatol® (felbamate) Oral Suspension 600 mg/5 mL are sorbitol, glycerin, microcrystalline cellulose, carboxymethylcellulose sodium, simethicone, polysorbate 80, methylparaben, saccharin sodium, propylparaben, FD&C Yellow No. 6, FD&C Red No. 40, flavorings, and purified water.
Mechanism of Action:
The mechanism by which felbamate exerts its anticonvulsant activity is unknown, but in animal test systems designed to detect anticonvulsant activity, felbamate has properties in common with other marketed anticonvulsants. Felbamate is effective in mice and rats in the maximal electroshock test, the subcutaneous pentylenetetrazol seizure test, and the subcutaneous picrotoxin seizure test. Felbamate also exhibits anticonvulsant activity against seizures induced by intracerebroventricular administration of glutamate in rats and N-methyl-D,L-aspartic acid in mice. Protection against maximal electroshock-induced seizures suggests that felbamate may reduce seizure spread, an effect possibly predictive of efficacy in generalized tonic-clonic or partial seizures. Protection against pentylenetetrazol-induced seizures suggests that felbamate may increase seizure threshold, an effect considered to be predictive of potential efficacy in absence seizures.
Receptor-binding studies in vitro indicate that felbamate has weak inhibitory effects on GABA-receptor binding, benzodiazepine receptor binding, and is devoid of activity at the MK-801 receptor binding site of the NMDA receptor-ionophore complex. However, felbamate does interact as an antagonist at the strychnine-insensitive glycine recognition site of the NMDA receptor-ionophore complex. Felbamate is not effective in protecting chick embryo retina tissue against the neurotoxic effects of the excitatory amino acid agonists NMDA, kainate, or quisqualate in vitro.
The monocarbamate, p-hydroxy, and 2-hydroxy metabolites were inactive in the maximal electroshock-induced seizure test in mice. The monocarbamate and p-hydroxy metabolites had only weak (0.2 to 0.6) activity compared with felbamate in the subcutaneous pentylenetetrazol seizure test. These metabolites did not contribute significantly to the anticonvulsant action of felbamate.
What is the most important information I should know about Felbatol?
Do not stop taking Felbatol without first talking to your healthcare provider.
Stopping Felbatol suddenly can cause serious problems.
Felbatol can cause serious side effects, including:
1. Felbatol may cause serious blood problems that may be life-threatening.
Call your healthcare provider right away if you have any of the following symptoms:
- Fever, sore throat or other infections that come and go or do not go away
- Frequent infections or an infection that does not go away
- Easy bruising
- Red or purple spots on your body
- Bleeding gums or nose bleeds
- Severe fatigue or weakness
2. Liver problems that may be life-threatening. Call your healthcare provider right away if you have any of these symptoms:
- yellowing of your skin or the whites of your eyes (jaundice)
- dark urine
- nausea or vomiting
- loss of appetite
- pain on the right side of your stomach (abdomen)
3. Like other antiepileptic drugs, Felbatol may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call your healthcare provider right away if you have any of these symptoms,
especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop Felbatol without first talking to a healthcare provider.
Stopping Felbatol suddenly can cause serious problems. You should talk to your health care provider before stopping. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures.
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
Who should not take Felbatol?
Do not take Felbatol if you:
- are allergic to felbamate, carbamates or any of the ingredients in Felbatol. See the end of this Medication Guide for a complete list of ingredients in Felbatol.
- have or have had blood problems
- have or have had liver problems
What should I tell my healthcare provider before taking Felbatol?
Before you take Felbatol, tell your healthcare provider if you:
- have kidney problems
- have or have had depression, mood problems, or suicidal thoughts or behavior
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if Felbatol can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking Felbatol. You and your healthcare provider will decide if you should take Felbatol while you are pregnant.
- If you become pregnant while taking Felbatol, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic medicine during pregnancy. You can enroll in this registry by calling 1-888-233-2334.
- are breastfeeding or plan to breastfeed. Felbatol may pass into your breast milk. You and your healthcare provider should decide if you should take Felbatol while you breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Taking Felbatol with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take Felbatol?
- Take Felbatol exactly as your healthcare provider tells you. Your healthcare provider will tell you how much Felbatol to take and when to take it.
- Your healthcare provider may change your dose of Felbatol. Do not change your dose of Felbatol without talking to your healthcare provider.
- Because of the risk of serious blood and liver problems, your healthcare provider may do blood tests before you start and while you take Felbatol.
- If you take too much Felbatol, call your healthcare provider or local Poison Control Center right away.
- Do not stop Felbatol without first talking to your healthcare provider.
What should I avoid while taking Felbatol?
- Felbatol can cause drowsiness and dizziness. Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking Felbatol, until you talk with your doctor. Taking Felbatol with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
What are the possible side effects of Felbatol?
See “What is the most important information I should know about Felbatol?”
Felbatol may cause serious side effects including:
The most common side effects of Felbatol include:
- weight loss
- vomiting
- trouble sleeping
- nausea
- dizziness
- sleepiness
- headache
- double-vision
- changes in the way that food tastes
These are not all the possible side effects of Felbatol. For more information, ask your healthcare provider or pharmacist.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Felbatol
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Felbatol for a condition for which it was not prescribed. Do not give Felbatol to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Felbatol. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Felbatol that is written for health professionals.
How should I store Felbatol?
- Store Felbatol at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Felbatol and all medicines out of the reach of children.
What are the ingredients in Felbatol?
Active Ingredient: felbamate
Inactive ingredients:
Tablet: starch, microcrystalline cellulose, croscarmellose sodium, lactose, magnesium stearate, FD&C yellow No. 6, D&C Yellow No. 10, and FD&C Red No. 40 (600 mg tablets only).
Suspension: sorbitol, glycerin, microcrystalline cellulose, carboxymethylcellulose sodium, simethicone, polysorbate 80, methylparaben, saccharain sodium, propylparaben, FD&C Yellow No. 6, FD&C Red No. 40, flavorings, and purified water.
Label
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 100-COUNT BOTTLE, 400 MG TABLET
- NDC 0037-0430-01
100 Tablets
Felbatol®
(felbamate)
Tablets 400 mg
Rx Only
MEDA
Meda Pharmaceuticals®
Somerset, New Jersey 08873-4120Usual Dosage: For full prescribing
information, see accompanying
package insert.
Store at controlled room temperature
20°-25°C (68°-77°F).
Dispense the accompanying
Medication Guide to each patient.
Dispense in a tight container. - Manufactured for:
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120 - LB-024G5-10 Rev. 5/2018
400934-01

![]()
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 8 FL OZ (237 ML) BOTTLE, 600 MG SUSPENSION
- NDC 0037-0442-67
8 fl oz (237 mL)
Felbatol®
(felbamate)
Oral Suspension
Each 5 mL contains
600 mg felbamate
Rx Only
SHAKE WELL
MEDA
PHARMACEUTICALS®
LB-024F5-12 141232-0821
Usual Dosage: For full prescribing information, see
accompanying package insert.
Store at controlled room temperature 20°-25°C (68°-77°F).
Dispense the accompanying Medication Guide to each
patient.
Dispense in a tight container.
Manufactured for:
Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120

SRC: NLM .
