Faslodex
Generic name: fulvestrant
Drug classes: Estrogen receptor antagonists, Hormones / antineoplastics
Medically reviewed by A Ras MD.
What is Faslodex?
Faslodex is a prescription medicine used to treat advanced breast cancer or breast cancer that has spread to other parts of the body (metastatic).
Faslodex may be used alone, if you have gone through menopause, and your advanced breast cancer is hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative and has not been previously treated with endocrine therapy or HR-positive and has progressed after endocrine therapy.
Faslodex may be used in combination with ribociclib, if you have gone through menopause, and your advanced or metastatic breast cancer is HR-positive and HER2-negative, and has not been previously treated with endocrine therapy or has progressed after endocrine therapy.
Faslodex may be used in combination with palbociclib or abemaciclib if your advanced or metastatic breast cancer is HR-positive and HER2-negative, and has progressed after endocrine therapy.
When Faslodex is used in combination with palbociclib, abemaciclib, or ribociclib, also read the Patient Information for the prescribed product.
It is not known if Faslodex is safe and effective in children.
It is not known if Faslodex is safe and effective in people with severe liver problems.
Who should not take Faslodex?
Do not receive Faslodex if you have had an allergic reaction to fulvestrant or any of the ingredients in Faslodex. See the end of this page for a list of the ingredients in Faslodex.
Symptoms of an allergic reaction to Faslodex may include:
- itching or hives
- swelling of your face, lips, tongue, or throat
- trouble breathing
Description
FASLODEX® (fulvestrant) injection for intramuscular administration is an estrogen receptor antagonist. The chemical name is 7-alpha-[9-(4,4,5,5,5-penta fluoropentylsulphinyl) nonyl]estra-1,3,5-(10)- triene-3,17-beta-diol. The molecular formula is C32H47F5O3S and its structural formula is:
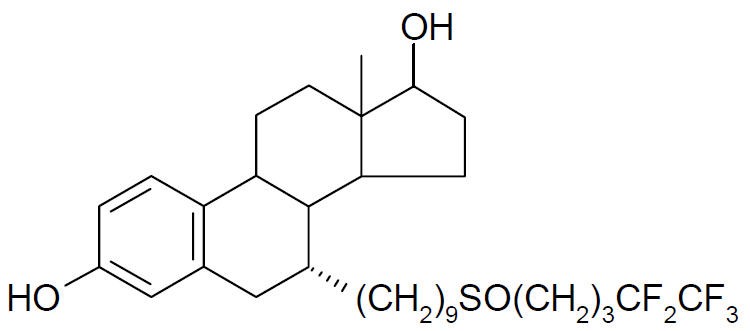
Fulvestrant is a white powder with a molecular weight of 606.77. The solution for injection is a clear, colorless to yellow, viscous liquid.
Each injection contains as inactive ingredients: 10% w/v Alcohol, USP, 10% w/v Benzyl Alcohol, NF, and 15% w/v Benzyl Benzoate, USP, as co-solvents, and made up to 100% w/v with Castor Oil, USP as a co-solvent and release rate modifier.
Mechanism of Action
Many breast cancers have estrogen receptors (ER) and the growth of these tumors can be stimulated by estrogen. Fulvestrant is an estrogen receptor antagonist that binds to the estrogen receptor in a competitive manner with affinity comparable to that of estradiol and downregulates the ER protein in human breast cancer cells.
In vitro studies demonstrated that fulvestrant is a reversible inhibitor of the growth of tamoxifen-resistant, as well as estrogen-sensitive human breast cancer (MCF-7) cell lines. In in vivo tumor studies, fulvestrant delayed the establishment of tumors from xenografts of human breast cancer MCF-7 cells in nude mice. Fulvestrant inhibited the growth of established MCF-7 xenografts and of tamoxifen-resistant breast tumor xenografts.
Fulvestrant showed no agonist-type effects in in vivo uterotrophic assays in immature or ovariectomized mice and rats. In in vivo studies in immature rats and ovariectomized monkeys, fulvestrant blocked the uterotrophic action of estradiol. In postmenopausal women, the absence of changes in plasma concentrations of FSH and LH in response to fulvestrant treatment (250 mg monthly) suggests no peripheral steroidal effects.
What should I tell my healthcare provider before taking Faslodex?
Before receiving Faslodex, tell your healthcare provider about all of your medical conditions, including if you:
- have a low level of platelets in your blood or bleed easily.
- have liver problems.
- are pregnant or plan to become pregnant. Faslodex can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider may perform a pregnancy test within 7 days before you start Faslodex.
- You should use effective birth control during treatment with Faslodex and for one year after the last dose of Faslodex.
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with Faslodex.
- are breastfeeding or plan to breastfeed. It is not known if Faslodex passes into your breast milk. Do not breastfeed during your treatment with Faslodex and for one year after the final dose of Faslodex. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Faslodex may affect the way other medicines work, and other medicines may affect how Faslodex works.
Especially tell your healthcare provider if you take a blood thinner medicine.
How should I take Faslodex?
- Your healthcare provider will give you Faslodex by injection into the muscle of each buttock.
- Your healthcare provider may change your dose of Faslodex if needed.
What are the possible side effects of Faslodex?
Faslodex may cause serious side effects, including:
- Injection site related nerve damage. Call your healthcare provider if you develop any of the following symptoms in your legs following a Faslodex injection:
- numbness
- tingling
- weakness
The most common side effects of Faslodex include:
- injection site pain
- nausea
- muscle, joint, and bone pain
- headache
- back pain
- tiredness
- pain in arms, hands, legs, or feet
- hot flashes
- vomiting
- loss of appetite
- weakness
- cough
- shortness of breath
- constipation
- increased liver enzymes
- diarrhea
Faslodex may cause fertility problems in males and females. Talk to your healthcare provider if you plan to become pregnant.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects with Faslodex. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Faslodex
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Faslodex that is written for health professionals.
How should I store Faslodex?
- Refrigerate, 2°-8°c (36°-46°f).
- To protect from light, store in the original carton until time of use.
What are the ingredients in Faslodex?
Active ingredient: fulvestrant.
Inactive ingredients: alcohol, benzyl alcohol, benzyl benzoate, and castor oil.
Label
PACKAGE/LABEL DISPLAY PANEL – 250 MG/5 ML (50 MG/ML)
|
FASLODEX® fulvestrant injection 250 mg/5 mL (50 mg/mL) For Intramuscular Use Only AstraZeneca |
NDC 0310-0720-10 This carton contains a total of 500 mg fulvestrant in TWO single-dose prefilled syringes each containing 250 mg/5 mL, and two Safety Glide™ shielding intramuscular injection needles. Discard each syringe after use. Both single-dose prefilled syringes must be administered to receive the 500 mg dose. REFRIGERATE, 2-8°C (36-46°F). TO PROTECT FROM LIGHT, STORE IN THE ORIGINAL CARTON UNTIL TIME OF USE. Rx only Contains 2 single-dose prefilled syringes. |

SRC: NLM .
