Faricimab-svoa
- Generic name: faricimab-svoa
- Dosage form: injection, solution
- Brand name: Vabysmo
Medically reviewed by A Rash MD. Last updated on March 24, 2022.
What is Faricimab-svoa
Faricimab-svoa side effects are the unwanted reactions that may be experienced when using this drug.
Faricimab-svoa is a bispecific immunoglobulin G1 (IgG1) antibody that binds to both VEGF-A and angiopoietin-2 (Ang-2). Selected point mutations in the fragment crystallizable (Fc) region of faricimab were used to eliminate binding interactions with Fc and FcRn receptors. Faricimab-svoa has a total molecular weight of about 149 kDa and is made using mammalian Chinese Hamster Ovary (CHO) cell culture and recombinant DNA technology.
Faricimab-svoa injection is a single-dose glass vial containing a sterile, transparent to opalescent, colorless to brownish-yellow solution for intravitreal administration. Each single-dose vial contains 6 mg faricimab-svoa, L-histidine (155 mcg), L-methionine (52.2 mcg), polysorbate 20 (20 mcg), sodium chloride (73.1 mcg), D-sucrose (2.74 mg), and Water for Injection, adjusted to pH 5.5 with acetic acid. There is no antimicrobial preservative in the product.¶
Mechanism of action
Faricimab-svoa is a humanized bispecific antibody that works by binding to VEGF-A and Ang-2 and inhibiting two pathways. Faricimab reduces endothelial cell proliferation, neovascularization, and vascular permeability by blocking VEGF-A. Faricimab is hypothesized to increase vascular stability and desensitize blood arteries to the effects of VEGF-A by suppressing Ang-2. In certain patients with nAMD and DME, Ang-2 levels are elevated. It is still unknown how much Ang-2 inhibition contributes to the therapy impact and clinical response in nAMD and DME.
Warnings and precautions
Endophthalmitis as well as Retinal Detachment
- Intravitreal injections have been linked with retinal detachments and endophthalmitis. The correct aseptic techniques for injections must be employed for administering Faricimab-svoa. Patients are advised to notify any signs of retinal detachment, or endophthalmitis promptly so that they can receive timely and prompt treatment.
Increase in the Intraocular Pressure
- Increases in the intraocular tension (IOP) have been observed within 60 minutes after intravitreal injections, including Faricimab-svoa. IOP and perfusion of the optic nerve head must be monitored and controlled appropriately.
Thromboembolic Events
- While there was a small incidence of arterial thromboembolic events (ATEs) that were observed within the Faricimab-svoa clinical studies, however, there is a possibility of ATEs after intravitreal treatment with VEGF inhibitors. Acute thromboembolic events are defined as strokes that are not fatal or myocardial infarction that is not fatal, or vascular deaths (including deaths due to unknown causes).
- The frequency of reported ATEs in the nAMD study at the beginning of the study was 11% (7 from 664) for patients receiving Faricimab-svoa in comparison to 1percent (6 from 662) in patients who were treated with Aflibercept.
- The rate of occurrence ATEs within the DME studies in the first year was percent (25 in 1,262) in patients who were treated with Faricimab-svoa, compared to 2percent (14 from 625) for patients receiving Aflibercept.
Dosing and administration
General Dosing Information
For intravitreal injection. Faricimab-svoa should be administered by a certified doctor. Each vial must be used to treat the condition of one eye.
Neovascular (wet) Age-Related Macular Degeneration (nAMD)
- The dose recommended of Faricimab-svoa can be 6 mg (0.05 120 mg/mL of solution) administered via intravitreal injection every four weeks (approximately every 28+- 7 days, every month) for the initial 4 doses. Then, optical coherence tomography as well as visual acuity tests at 8 and 12 weeks to determine if it is appropriate to administer the dose of 6 mg via intravitreal injections on any of the three following regimens that include: 1)) Weeks 28 and 44; 2) Weeks 24, 36 and 48;) Weeks 24 36, 36 and 48; or) Weeks 20, 28 36, and 44. While additional efficacy wasn’t observed in all patients who received Faricimab-svoa was administered every 4 weeks, compared with every eight weeks. Some patients could require every 4 weeks (monthly) doses following the initial 4 doses. Patients should be monitored frequently.
Diabetic Macular Edema (DME)
- Faricimab-svoa is suggested to be administered using either of the following dose regimens: 1)) 6.25 mg (0.05 120 mg/mL of solution) given via intravitreal injections every 4 weeks (approximately every 28 days plus7 days – 7 days, every month) for at minimum 4 doses. If, after at minimum four doses, the resolution of edema as measured by CST, the thickness of the subfield central to (CST) of the macula as determined using optical coherence tomography has been attained, then the frequency of dosing can be altered by extending up to four weeks of interval increments, or reductions of up to eight-week interval increments, based on the CST as well as visual acuity assessments until week 52. 2.) the dose of 6 mg Faricimab-svoa can be administered each 4 weeks during the initial six doses, then followed by a dose of 6 mg by intravitreal injections at intervals of eight weeks (2 months) for the following 28 weeks. Although the additional effectiveness was not evident in the majority of patients who received Faricimab-svoa was administered every 4 weeks as opposed to every eight weeks. Some patients could require every 4 weeks (monthly) doses following the initial 4 doses. Patients should be monitored often.
Preparation for Administration
Injection Procedure
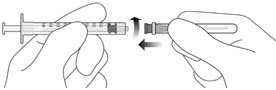
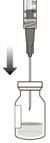
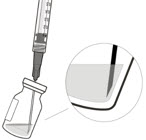
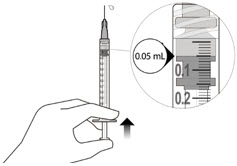
- The intravitreal injection procedure should be done under aseptic conditions. This requires the use of hand disinfection for surgical procedures gloves that are sterile, a sterile drape, an eyelid speculum made of sterile (or equivalent), and the availability of paracentesis equipment that is sterile (if needed). Sufficient anesthesia, as well as a broad-spectrum microbicide, must be administered prior to injection. Then, slowly inject until the stopper of the rubber reaches the point at which the syringe is able to give the dose of 0.05 milliliters. Verify the delivery of the complete dose by ensuring that the stopper’s rubber is at the top of the barrel.
- Any unopened medicinal product or waste materials should be removed in line with local regulations.
- Following the intravitreal injection, patients must be monitored for an increase in intraocular pressure. The appropriate monitoring could include an examination of perfusion to the optic nerve’s head or tonometry. If needed, a sterilized paracentesis needle is required. After intravitreal injections patients are instructed to report any symptoms that may be indicative of retinal detachment or endophthalmitis (e.g. vision loss eye pain, eyes that are red blurring of vision, photophobia) promptly. Each syringe is to be used for the treatment of one eye. If the eye on the opposite side is in need of treatment, a fresh needle should be utilized as well as the sterile field. the syringe, gloves eyelid speculum, filters, and needles for injection should be replaced prior to when Faricimab-svoa can be administered in the opposite eye.
Dosage and strength
Injection: 120 mg/mL clear to opalescent, colorless to brownish-yellow solution in a single-dose vial.
Faricimab-svoa side effects
infrequent side effects
- hemorrhage in vitreous
- a blood clot in an artery
- the detachment of retinal pigment epithelium
- eye troubles
- eye pain
- eye inflammation
- eye itching
- streaks or black specks due to floating eye matter
Rare side effects
- inflammation on the inside of the eye
- a tear of the retina of the eye
- affixation from the retina of the eye
- increased pressure in the eye
- abrasions of the cornea
- blurred vision
- headache, nausea
- a decrease in the sharpness of vision is referred to as reduced visual acuity.
- excessive watery eyes
- red eyes
- eye itches
- the feeling that something is in the eye
Patient Counseling
Patients should be informed that they are at risk of getting endophthalmitis in the days following Faricimab-svoa treatment. Advise the patient to consult an ophthalmologist right once if the eye gets red, sensitive to light, painful, or develops a change in vision.
Following intravitreal injection of Faricimab-svoa and the concomitant eye tests, patients may experience transitory vision abnormalities. Patients should not drive or operate machines until their vision has improved adequately.
Label
Vabysmo™
(faricimab-svoa)
Injection
NDC 50242-096-01
6 mg (0.05 mL of 120 mg/mL solution)
For Intravitreal Injection. Single-Dose Vial.
Discard Unused Portion.
1 single-dose vial
1 transfer filter needle
Rx only
Genentech