Ezallor
Generic name: rosuvastatin
Brand names: Crestor, Ezallor Sprinkle
Drug class: Statins
Medically reviewed by A Ras MD.
What is Ezallor Sprinkle?
Ezallor Sprinkle is a prescription medicine used in adults along with diet to lower the level of your “bad” cholesterol (LDL), and lower the level of fat in your blood (triglycerides).
Pediatric use information for patients 7 to 17 years of age is approved for AstraZeneca’s Crestor (rosuvastatin calcium) tablets. However, due to AstraZeneca’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
It is not known if Ezallor Sprinkle is safe and effective in people who have Fredrickson Type I and V dyslipidemias.
Description
Rosuvastatin calcium is a synthetic lipid-lowering agent for oral administration.
The chemical name for rosuvastatin calcium is bis[(E)-7-[4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl]-(3R,5S)-3,5-dihydroxyhept-6-enoic acid] calcium salt with the following structural formula:
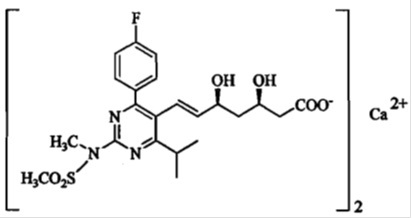
The molecular formula for rosuvastatin calcium is (C22H27FN3O6S)2·Ca and the molecular weight is 1,001.14. Rosuvastatin calcium is off-white to light yellow amorphous powder that is slightly soluble in water and methanol, and insoluble in ethanol. Rosuvastatin calcium is a hydrophilic compound with a partition coefficient (octanol/water) of 0.13 at pH of 7.
Each rosuvastatin capsule for oral administration contains 5 mg, 10 mg, 20 mg, or 40 mg of rosuvastatin (present as 5.198 mg, 10.395 mg, 20.790 mg, or 41.580 mg of rosuvastatin calcium) in the form of granules with the following inactive ingredients: microcrystalline cellulose, crospovidone, mannitol, magnesium oxide, ferric oxide, sodium citrate, hypromellose, polyethylene glycol 4000, and silicon dioxide. The capsule shells have the following inactive ingredients: gelatin, titanium dioxide, water, and sodium lauryl sulfate. Additionally following colorants are used in capsules: FD&C Red 40 (5 mg), FD&C Blue 1 (5 mg, 10 mg, 20 mg), D&C Red 28 (5 mg, 10 mg), FD&C Red 3 (20 mg), and FD&C Green 3 (40 mg). The imprinting black ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, purified water, and potassium hydroxide.
Mechanism of Action
Rosuvastatin is a selective and competitive inhibitor of HMG-CoA reductase, the rate-limiting enzyme that converts 3-hydroxy-3-methylglutaryl coenzyme A to mevalonate, a precursor of cholesterol. In vivo studies in animals, and in vitro studies in cultured animal and human cells have shown rosuvastatin to have a high uptake into, and selectivity for, action in the liver, the target organ for cholesterol lowering. In in vivo and in vitro studies, rosuvastatin produces its lipid-modifying effects in two ways. First, it increases the number of hepatic LDL receptors on the cell-surface to enhance uptake and catabolism of LDL. Second, rosuvastatin inhibits hepatic synthesis of VLDL, which reduces the total number of VLDL and LDL particles.
Who should not take Ezallor Sprinkle?
Do not take Ezallor Sprinkle if you:
- are allergic to rosuvastatin calcium or any of the ingredients in Ezallor Sprinkle. See the end of this guide for a complete list of ingredients in Ezallor Sprinkle.
- have liver problems.
- are pregnant or think you may be pregnant or are planning to become pregnant. Ezallor Sprinkle may harm your unborn baby. If you become pregnant, stop taking Ezallor Sprinkle and call your doctor right away. If you are not planning to become pregnant you should use effective birth control (contraception) while you are taking Ezallor Sprinkle.
- are breastfeeding. Ezallor Sprinkle can pass into your breast milk and may harm your baby.
What should I tell my healthcare provider before taking Ezallor Sprinkle?
Before taking Ezallor Sprinkle, tell your doctor about all of your medical conditions, including if you:
- have unexplained muscle aches or weakness.
- have or have had kidney problems.
- have had liver problems.
- drink more than 2 glasses of alcohol daily.
- have thyroid problems.
- are 65 years of age or older.
- are of Asian descent.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Talk to your doctor before you start taking any new medicines.
Taking Ezallor Sprinkle with certain other medicines may affect each other causing side effects. Ezallor Sprinkle may affect the way other medicines work, and other medicines may affect how Ezallor Sprinkle works.
Especially tell your doctor if you take:
- cyclosporine (a medicine for your immune system)
- gemfibrozil (a fibric acid medicine for lowering cholesterol)
- anti-viral medicines including HIV or hepatitis C protease inhibitors (such as lopinavir, ritonavir, fosamprenavir, tipranavir, atazanavir, or simeprevir)
- certain anti-fungal medicines (such as itraconazole, ketoconazole and fluconazole)
- coumarin anticoagulants (medicines that prevent blood clots, such as warfarin)
- niacin or nicotinic acid
- fibric acid derivatives (such as fenofibrate)
- colchicine (a medicine used to treat gout)
Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Know all of the medicines you take. Keep a list of them to show your doctor and pharmacist when you get new medicine.
How should I take Ezallor Sprinkle?
- Take Ezallor Sprinkle exactly as your doctor tells you to take it.
- Take Ezallor Sprinkle, by mouth, 1 time each day. Swallow the capsule whole.
- Do not crush or chew Ezallor Sprinkle.
- Ezallor Sprinkle can be taken at any time of day, with or without food.
- If you have trouble swallowing a whole capsule, you can open the capsule and take the contents with soft food (applesauce, or chocolate- or vanilla-flavored pudding). See the “Instructions for Use” that comes with Ezallor Sprinkle for instructions on how to take Ezallor Sprinkle with soft food (applesauce, or chocolate- or vanilla-flavored pudding).
- See the “Instructions for Use” that comes with Ezallor Sprinkle for instructions on how to mix and give Ezallor Sprinkle through a nasogastric tube.
- Do not change your dose or stop Ezallor Sprinkle without talking to your doctor.
- Your doctor may do blood tests to check your cholesterol levels before and during your treatment with Ezallor Sprinkle. Your doctor may change your dose of Ezallor Sprinkle if needed.
- Your doctor may start you on a cholesterol lowering diet before giving you Ezallor Sprinkle. Stay on this diet when you take Ezallor Sprinkle.
- Wait at least 2 hours after taking Ezallor Sprinkle to take an antacid that contains a combination of aluminum and magnesium hydroxide.
- If you miss a dose of Ezallor Sprinkle, take it as soon as you remember. Do not take 2 doses of Ezallor Sprinkle within 12 hours of each other.
- If you take too much Ezallor Sprinkle or overdose, call your doctor or go to the nearest hospital emergency room right away.
What are the possible side effects of Ezallor Sprinkle?
Ezallor Sprinkle may cause serious side effects, including:
- Muscle pain, tenderness and weakness (myopathy). Muscle problems, including muscle breakdown, can be serious in some people and rarely cause kidney damage that can lead to death. Tell your doctor right away if:
- you have unexplained muscle pain, tenderness, or weakness, especially if you have a fever or feel more tired than usual, while you take Ezallor Sprinkle.
- you have muscle problems that do not go away even after your doctor has told you to stop taking Ezallor Sprinkle. Your doctor may do further tests to diagnose the cause of your muscle problems.
Your chances of getting muscle problems are higher if you:
-
- are taking certain other medicines while you take Ezallor Sprinkle
- are 65 years of age or older
- have thyroid problems (hypothyroidism) that are not controlled
- have kidney problems
- are taking higher doses of Ezallor Sprinkle
- Liver problems. Your doctor should do blood tests to check your liver before you start taking Ezallor Sprinkle and if you have symptoms of liver problems while you take Ezallor Sprinkle. Call your doctor right away if you have any of the following symptoms of liver problems:
- feel unusually tired or weak
- loss of appetite
- upper belly pain
- dark urine
- yellowing of your skin or the whites of your eyes
The most common side effects may include: headache, muscle aches and pains, abdominal (stomach) pain, weakness, and nausea.
Additional side effects that have been reported with Ezallor Sprinkle include memory loss and confusion.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Ezallor Sprinkle. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Ezallor Sprinkle
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Ezallor Sprinkle for a condition for which it was not prescribed. Do not give Ezallor Sprinkle to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about Ezallor Sprinkle that is written for health professionals.
How should I store Ezallor Sprinkle?
- Store Ezallor Sprinkle at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Store in a dry place.
- Safely throw away medicine that is out of date or no longer needed.
Keep Ezallor Sprinkle and all medicines out of the reach of children.
What are the ingredients in Ezallor Sprinkle?
Active Ingredient: rosuvastatin as rosuvastatin calcium
Inactive Ingredients: microcrystalline cellulose, crospovidone, mannitol, magnesium oxide, ferric oxide, sodium citrate, hypromellose, polyethylene glycol 4000, and silicon dioxide.
Capsule shell: gelatin, titanium dioxide, water, sodium lauryl sulfate and the following colorants: FD&C Red 40 (5 mg), FD&C Blue 1 (5 mg, 10 mg, 20 mg), D&C Red 28 (5 mg, 10 mg), FD&C Red 3 (20 mg), and FD&C Green 3 (40 mg).The imprinting black ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, purified water, and potassium hydroxide.
Label
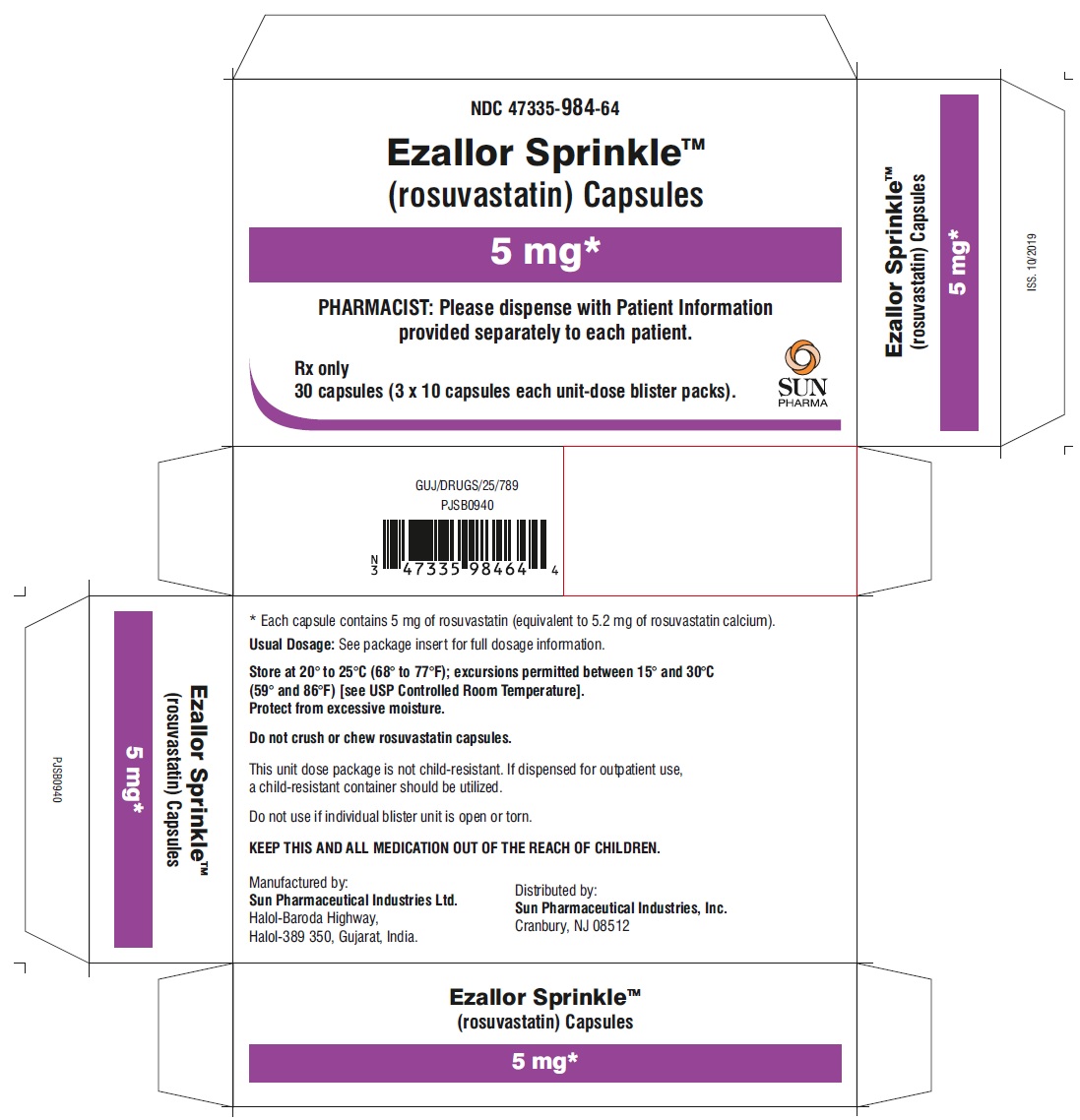
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL – CARTON – 5 MG
- NDC 47335-984-64
- Ezallor SprinkleTM
(rosuvastatin) Capsules - 5 mg
- PHARMACIST: Please dispense with Patient Information provided separately to each patient.
- Rx only
- 30 (3 × 10) Unit-Dose Capsules
- SUN PHARMA
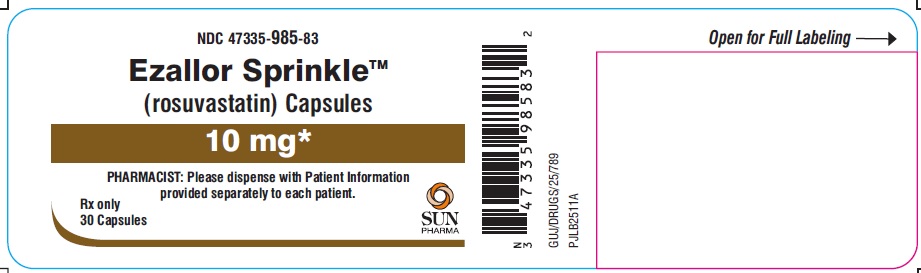
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL – LABEL – 10 MG
- NDC 47335-985-83
- Ezallor SprinkleTM
(rosuvastatin) Capsules - 10 mg
- PHARMACIST: Please dispense with Patient Information provided separately to each patient.
- Rx only
- 30 Capsules
- SUN PHARMA
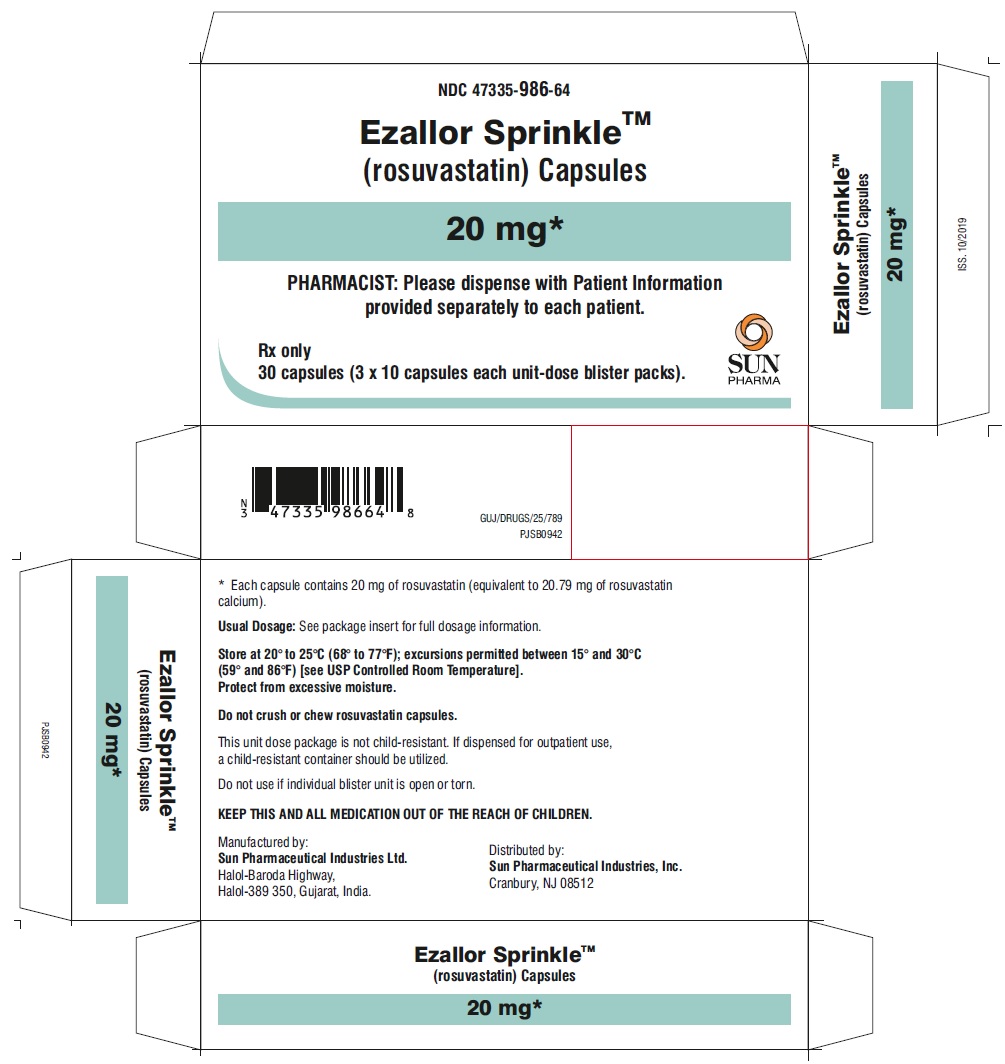
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL – CARTON – 20 MG
- NDC 47335-986-64
EzallorSprinkleTM
(rosuvastatin) Capsules
20 mg
PHARMACIST: Please dispense with Patient Information provided separately to each patient.
Rx only
30 (3 × 10) Unit-Dose Capsules
SUN PHARMA
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL – LABEL – 40 MG
- NDC 47335-987-83
Ezallor SprinkleTM
(rosuvastatin) Capsules
40 mg*
PHARMACIST: Please dispense with Patient Information provided separately to each patient.
Rx only
30 Capsules
SUN PHARMA

