Extavia
Generic name: interferon beta-1b
Drug class: Interferons
Medically reviewed by A Ras MD.
What is Extavia?
Extavia is a prescription medicine used to treat relapsing forms of multiple sclerosis (MS), to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. Extavia is similar to certain interferon proteins that are produced in the body. It is not known if Extavia is safe and effective in children.
Description
Interferon beta-1b is a purified, sterile, lyophilized protein product produced by recombinant DNA techniques. Interferon beta-1b is manufactured by bacterial fermentation of a strain of Escherichia coli that bears a genetically engineered plasmid containing the gene for human interferon betaser17. The native gene was obtained from human fibroblasts and altered in a way that substitutes serine for the cysteine residue found at position 17. Interferon beta-1b has 165 amino acids and an approximate molecular weight of 18,500 daltons. It does not include the carbohydrate side chains found in the natural material.
The specific activity of EXTAVIA is approximately 32 million international units (IU)/mg interferon beta-1b. Each vial contains 0.3 mg of interferon beta-1b. The unit measurement is derived by comparing the antiviral activity of the product to the World Health Organization (WHO) reference standard of recombinant human interferon beta. Mannitol, USP and Albumin (Human), USP (15 mg each/vial) are added as stabilizers.
EXTAVIA (interferon beta-1b) for injection is a sterile, preservative-free, white to off-white lyophilized powder, for subcutaneous injection after reconstitution with the diluent supplied (0.54% Sodium Chloride Solution, USP). Each vial contains Albumin (Human), USP (15 mg) and Mannitol, USP (15 mg).
Mechanism of Action
The mechanism of action of EXTAVIA (interferon beta-1b) in patients with MS is unknown.
What is the most important information I should know about Extavia?
Extavia can cause serious side effects, including:
- liver problems including liver failure. Symptoms of liver problems may include:
- serious allergic reactions. Serious allergic reactions can happen quickly and may happen after your first dose of Extavia or after you have taken Extavia many times. Symptoms may include: difficulty breathing, swallowing, swelling of the mouth or tongue, rash, itching, skin bumps
- depression or suicidal thoughts. Call your healthcare provider right away if you have any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- trouble sleeping (insomnia)
- hallucinations
- new or worse depression
- acting aggressive, being angry, or violent
- other unusual changes in behavior or mood
- new or worse anxiety
- acting on dangerous impulses
Who should not take Extavia?
Do not take Extavia if you are allergic to interferon beta-1b, to another interferon beta, to human albumin, or mannitol. See the end of this leaflet for a complete list of ingredients in Extavia.
What should I tell my healthcare provider before taking Extavia?
Before you take Extavia, tell your healthcare provider if you:
- have or have had depression (sinking feeling or sadness), anxiety (feeling uneasy, nervous, or fearful for no reason) or trouble sleeping
- have or have had liver problems
- have or have had blood problems such as bleeding or bruising easily, low red blood cells (anemia) or low white blood cells
- have or have had seizures
- have or have had heart problems
- have or have had an allergic reaction to rubber or latex. The rubber cap of the diluent pre-filled syringe contains natural rubber latex.
- are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if Extavia passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Extavia?
- See the Instructions for Use that at the end of this Medication Guide for complete information on how to use Extavia.
- Extavia is given by injection under your skin (subcutaneous injection) every other day.
- Take Extavia exactly as your healthcare provider tells you to take it.
- If your healthcare provider feels that you or someone else may give you the injections, then you or the other person should be trained by your healthcare provider in how to give an injection.
- Do not try to give yourself or have another person give you injections until you or both of you understand and are comfortable with how to prepare your dose and give the injection.
- You may be started on a lower dose when you first start taking Extavia. Your healthcare provider will tell you what dose of Extavia to use.
- Your healthcare provider may change your dose of Extavia. You should not change your dose without talking to your healthcare provider.
- If you miss a dose, you should take your next dose as soon as you remember or are able to take it. Your next injection should be taken about 48 hours (2 days) after that dose. Do not take Extavia on 2 consecutive days. If you accidentally take more than your prescribed dose, or take it on 2 consecutive days, call your healthcare provider right away.
- Always use a new, unopened vial of Extavia and pre-filled diluent syringe for each injection. Throw away any unused medicine. Do not re-use any vials, syringes, or needles.
- It is important for you to change your injection site each time you inject Extavia. This will lessen the chance of you having a serious skin reaction at the site where you inject Extavia. Avoid injecting Extavia into an area of skin that is sore, reddened, infected, or has other problems.
What are the possible side effects of Extavia?
Extavia may cause serious side effects. Call your healthcare provider right away if you have any of the serious side effects of Extavia, including:
- See “What is the most important information I should know about Extavia?”
- heart problems. Extavia may worsen heart problems, including congestive heart failure. Symptoms of heart problems may include:
- swollen ankles
- tightness in chest
- shortness of breath
- increased need to urinate at night
- decreased ability to exercise
- not being able to lay flat in bed
- fast heartbeat
- Injection-site problems. Serious skin reactions can happen in some people including areas of severe damage to skin and the tissue below the skin (necrosis). These reactions can happen anywhere you inject Extavia. Symptoms of injection site problems may include swelling, redness, or pain at the injection site, fluid drainage from the injection site, and breaks in your skin or blue-black skin discoloration
- flu-like symptoms. Extavia can cause flu-like symptoms including:
- fever
- chills
- tiredness
- sweating
- muscle aches when you first start to use it
These symptoms may decrease over time. Taking medicines for fever and pain relief on the days you are using Extavia may help decrease these symptoms.
- seizures. Some people have had seizures while taking Extavia, including people who have never had seizures before. It is not known if the seizures were related to their MS, to Extavia, or to a combination of both. If you have a seizure after taking Extavia, call your healthcare provider right away.
The most common side effects of Extavia include:
- low white blood cell count
- increases in your muscle tension
- problems sleeping
- increases in your liver enzymes
- pain
- stomach pain
- headache
- rash
- weakness
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Extavia. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Extavia
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Extavia for a condition for which it was not prescribed. Do not give Extavia to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Extavia. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Extavia that is written for health professionals. For more information, go to www.EXTAVIA.com or call the Extavia toll-free medical information line at 1-888-669-6682.
How should I store Extavia?
- Before mixing, store Extavia at room temperature between 68°F to 77°F (20°C to 25°C).
- Before mixing, Extavia may be stored for up to 3 months between 59°F to 86°F (15°C to 30°C).
- After mixing, you can refrigerate Extavia for up to 3 hours before using. Your Extavia must be used within 3 hours of mixing even if refrigerated.
- Do not freeze.
Keep Extavia and all medicines out of the reach of children.
What are the ingredients in Extavia?
Active ingredient: interferon beta-1b
Inactive ingredients: albumin (human), mannitol
Diluent contains sodium chloride solution.
Instructions for use for Extavia (interferon beta-1b)
If your doctor decides that you or a caregiver may be able to give your injections of Extavia at home, your doctor or nurse should instruct you on the right way to prepare and inject Extavia. To lower your risk of infection, it is important that you follow the technique that your doctor or nurse discussed with you to prepare and inject Extavia. Do not try to inject Extavia yourself until you have been shown by your doctor or nurse the right way to prepare and give the injections.
It is important for you to read, understand, and follow these instructions. Call your doctor if you or your caregiver has any questions about the right way to prepare or inject Extavia.
Important safety information
- The rubber cap on the diluent pre-filled syringe is made of natural rubber latex. Tell your doctor if you are allergic to rubber or latex.
- Do not leave the blister pack containing Extavia where others might tamper with it.
- Keep the blister pack containing Extavia out of the reach of children.
- Do not open the blister pack or take out any of the items until right before you are ready to use them.
- Do not use Extavia if the seal on the vial is broken. If the seal is broken, the product may not be safe for you to use.
- Do not use Extavia after the expiration date shown on the blister pack label or box (Figure 1). If it has expired, return the entire pack to the pharmacy.
Figure 1
- Do not use any of the items in the blister pack more than one time. See the section at the end of this leaflet, “Dispose of used syringes, needles, and vials.” Throw away any open and unused medicine.
Gather your supplies.
You will need the following supplies to get ready to give your injection of Extavia:
- A blister pack containing the following items (Figure 2)
- a vial of Extavia
- a pre-filled syringe of diluent (0.54% Sodium Chloride Solution)
- a vial adapter with a 27-gauge needle attached (in its own container)
- two (2) alcohol wipes
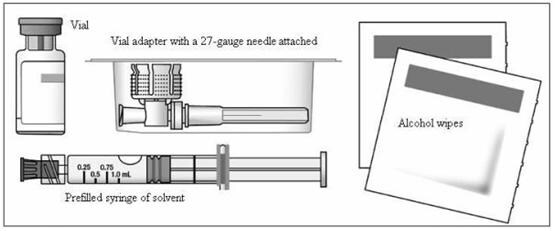
Figure 2
- a dry cotton ball and gauze
- a sharps disposal container (Figure 3). See the section “Dispose of used syringes, needles, and vials”
Figure 3
Prepare for self-injection
- Wash your hands well with soap and water.
- Open the blister pack by peeling off the label and take out all the items. Make sure the blister pack containing the vial adapter is sealed. Check to make sure the rubber cap on the diluent syringe is firmly attached.
- Turn the blister pack over, and place the vial in the well (vial holder) and place the pre-filled syringe in the U-shaped trough (Figure 4).
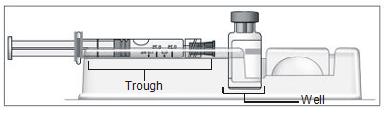
Figure 4
Mix Extavia
4. Remove the Extavia vial from the well and take the cap off the vial (Figure 5).
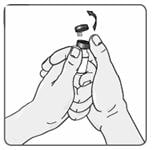
Figure 5
5. Place the vial back in the vial holder.
6. Use an alcohol wipe to clean the top of the vial (Figure 6). Wipe in one direction only.
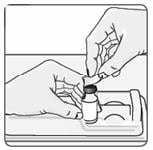
Figure 6
7. Leave the alcohol wipe on top of the vial until step 9 below.
8. Peel the label off the container with the vial adapter in it, but do not remove the vial adapter. The vial adapter is sterile, so do not touch it.
9. Remove the alcohol wipe from the top of the vial. Pick up the container that holds the vial adapter. Turn over the container keeping the vial adaptor inside. Put the adapter on top of the vial. Push down on the adapter until it pierces the rubber top of the vial and snaps in place (Figure 7). Lift the container off the vial adapter.
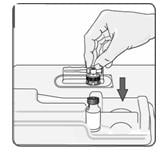
Figure 7
10. Remove the rubber cap from the pre-filled syringe using a twist and pull motion (Figure 8). Throw away the rubber cap.
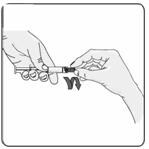
Figure 8
11. Remove the vial from the vial holder by grasping the vial. Do not touch any part of the vial adapter. Be careful not to pull the vial adapter off the top of the vial.
12. Connect the pre-filled syringe of diluent to the vial adapter by turning clockwise and tighten carefully (Figure 9).
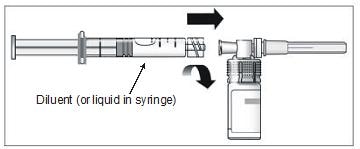
Figure 9
13. Slowly push the plunger of the pre-filled syringe all the way in. This will push all of the liquid from the syringe into the vial (Figure 10). Continue to hold the plunger while you mix Extavia with the liquid from the syringe. If you do not hold the plunger in, it may return to its original position after you let go.
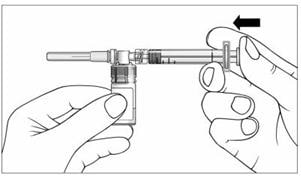
Figure 10
14. Gently swirl the vial to completely dissolve the white powder (Extavia). Do not shake. Shaking and even gentle mixing can cause foaming of the medicine. If there is foam, let the vial sit until the foam settles.
15. After the powder dissolves, look closely at the solution in the vial. Do not use the solution if it is not clear or colorless, or if it contains particles.
The injection should be given right away after you mix Extavia and let any foam in the solution settle. If you must wait for any reason before giving yourself the injection, you may refrigerate the medicine after you mix it. But you should use it within three hours.
16. With your thumb still pushing the plunger, turn the syringe and vial, so that the vial is on top (Figure 11).
17. Slowly pull the plunger back to withdraw the entire contents of the vial into the syringe.
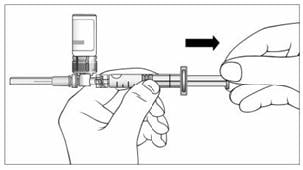
Figure 11
18. Turn the syringe so that the needle end is pointing up. Remove any air bubbles by tapping the outside of the syringe with your fingers (Figure 12). Slowly push the plunger to the 1 mL mark on the syringe or to the mark that matches the amount of Extavia prescribed by your doctor. If too much solution is pushed back into the vial, return to step 16.
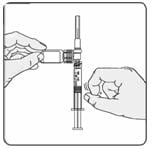
Figure 12
19. Remove the vial adapter and the vial from the syringe by twisting the vial adapter (Figure 13).
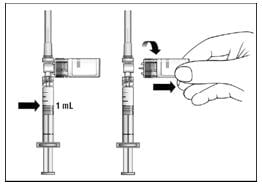
Figure 13
Choose an Injection Site
- Extavia is injected under the skin and into the fat layer between the skin and the muscles (subcutaneous tissue). The best areas for injection are where the skin is loose and soft and away from the joints, nerves, and bones. Do not use the area near your navel (belly button) or waistline. If you are very thin, use only the thigh or outer surface of the arm for injection.
- Choose a different site each time you give yourself an injection. Figure 14 shows different areas for giving injections. Do not inject in the same area for two injections in a row. Keep a record of your injections to help make sure you change (rotate) your injection sites. If there are any sites that are difficult for you to reach, you can ask someone who has been trained to give the injection to you.
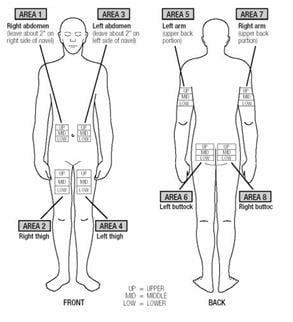
Figure 14
- Do not inject Extavia in a site where the skin is red, bruised, infected, or scabbed, has broken open, or has lumps, bumps, or pain. Tell your doctor if you find skin conditions like the ones mentioned here or any other unusual looking areas where you have been given injections.
Injecting Extavia
20. Using a circular motion, clean the injection site with an alcohol wipe, starting at the injection site and moving outward (Figure 15). Let the skin area air dry.
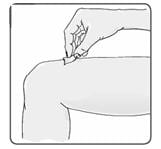
Figure 15
21. Remove the cap from the needle (Figure 16).
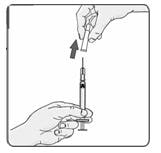
Figure 16
22. Gently pinch the skin around the site with your thumb and forefinger of the other hand (Figure 17). Insert the needle straight up and down into your skin at a 90˚ angle with a quick, dart-like motion.
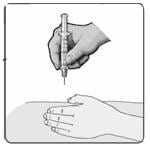
Figure 17
23. Once the needle is in your skin, slowly pull back on the plunger. If blood appears in the syringe, it means that you have entered a blood vessel. Do not inject Extavia. Withdraw the needle. Throw away the syringe and needle in your puncture-proof container. Do not use the same syringe or any of the other supplies that you used for this injection. Repeat the above steps to prepare your dose using a new blister pack. Choose and clean a new injection site.
24. If no blood appears in the syringe, slowly push the plunger all the way in until the syringe is empty (Figure 18). Remove the needle from the skin; then place a dry cotton ball or gauze pad over the injection site. Gently massage the injection site for a few minutes with the dry cotton ball or gauze pad. Throw away the syringe in your puncture-proof disposal container.
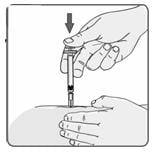
Figure 18
Dispose of used syringes, needles, and vials
- To prevent needle-stick injury and spread of infection, do not try to re-cap the needle.
- Place used needles, syringes, and vials in a closeable, puncture-resistant container. You may use a sharps container (such as a red biohazard container), a hard plastic container (such as a detergent bottle), or a metal container (such as an empty coffee can). Do not use glass or clear plastic containers. Ask your doctor for instructions on the right way to throw away (dispose of) the container. There may be state and local laws about how you should throw away used needles and syringes.
- Do not throw used needles, syringes, or vials in your household trash or recycle.
- Throw away any unused medicine. Do not save any unused Extavia for a future dose.
- Keep the disposal container, needles, syringes, and vials of Extavia out of the reach of children.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0078-0569-12
- NOVARTIS
- EXTAVIA®
- (interferon beta-1b)
- 0.3 mg per vial
- For subcutaneous use only
- Rx only
- Dispense the enclosed Medication Guide to each patient.
- Each blister pack contains:
- • one 1.2 mL prefilled single use sodium chloride 0.54% solution diluent syringe
- • one single dose vial for reconstitution containing Extavia
- • one vial adapter with attached 27-gauge needle
- • two alcohol prep pads
- Dispense in this unit-of-use container.
- 15 single dose
blister packs

SRC: NLM .
