Exkivity
Generic name: mobocertinib
Dosage form: capsules
Drug class: EGFR inhibitors
Medically reviewed by A Ras MD.
What is Exkivity?
Exkivity is a prescription medicine used to treat adults with non-small cell lung cancer (NSCLC) that has spread to other parts of the body (metastatic) and cannot be removed by surgery, and has a certain abnormal epidermal growth factor receptor (EGFR) gene, and whose disease has worsened while on or after chemotherapy that contains platinum.
Your healthcare provider will perform a test to make sure that Exkivity is right for you.
It is not known if Exkivity is safe and effective in children.
Description
Mobocertinib is a kinase inhibitor. The chemical name for mobocertinib succinate is propan-2-yl 2-[5-(acryloylamino)-4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxyanilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate succinate. The molecular formula is C32H39N7O4 + C4H6O4 (succinate salt) which corresponds to a molecular weight of 703.8 g/mol. Mobocertinib has no chiral centers. The chemical structure of mobocertinib succinate is shown below:
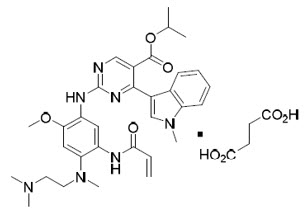
Mobocertinib succinate has a solubility of 152 mg/mL in pH 1.0 and >17.6 mg/mL in pH 6.8 solutions at 37°C.
EXKIVITY capsule for oral administration contains 40 mg mobocertinib equivalent to 48.06 mg mobocertinib succinate, with no inactive ingredients. The capsule shells contain gelatin and titanium dioxide. The printing ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water.
Mechanism of Action
Mobocertinib is a kinase inhibitor of the epidermal growth factor receptor (EGFR) that irreversibly binds to and inhibits EGFR exon 20 insertion mutations at lower concentrations than wild type (WT) EGFR. Two pharmacologically-active metabolites (AP32960 and AP32914) with similar inhibitory profiles to mobocertinib have been identified in the plasma after oral administration of mobocertinib. In vitro, mobocertinib also inhibited the activity of other EGFR family members (HER2 and HER4) and one additional kinase (BLK) at clinically relevant concentrations (IC50 values <2 nM).
In cultured cell models, mobocertinib inhibited the proliferation of cells driven by different EGFR exon 20 insertion mutation variants at 1.5- to 10-fold lower concentrations than WT-EGFR signaling inhibition.
In animal tumor implantation models, mobocertinib exhibited anti-tumor activity against xenografts with the EGFR exon 20 insertions NPH or ASV.
What is the most important information I should know about Exkivity?
Exkivity may cause serious side effects, including:
- Changes in the electrical activity of your heart called QTc prolongation and Torsades de Pointes. QTc prolongation can cause irregular heartbeats that can be life-threatening and may lead to death. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) and do blood tests to check your electrolytes before starting and during treatment with Exkivity. Tell your healthcare provider right away if you feel dizzy, lightheaded, faint or have an irregular heartbeat.
See “What are the possible side effects of Exkivity?” for more information about side effects.
What should I tell my healthcare provider before taking Exkivity?
Before taking Exkivity, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems, including a condition called long QTc syndrome
- have problems with your electrolytes, such as sodium, potassium, calcium or magnesium
- have lung or breathing problems other than lung cancer
- are pregnant or plan to become pregnant. Exkivity can harm your unborn baby.
- Females who are able to become pregnant:
- Your healthcare provider should do a pregnancy test before you start treatment with Exkivity.
- You should use an effective form of non-hormonal birth control during treatment and for 1 month after your last dose of Exkivity.
- Birth control pills (oral contraceptives) and other hormonal forms of birth control may not work as well during treatment with Exkivity.
- Talk to your healthcare provider about birth control methods that might be right for you during this time.
- Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with Exkivity.
- Males who have female partners who are able to become pregnant:
- You should use effective birth control during treatment and for 1 week after your last dose of Exkivity.
- Females who are able to become pregnant:
- are breastfeeding or plan to breastfeed. It is not known if Exkivity passes into your breast milk. Do not breastfeed during treatment and for 1 week after your last dose of Exkivity.
Tell your healthcare provider about all the medicines you take, including prescription medicines and over-the-counter medicines, vitamins, and herbal supplements. Tell your healthcare provider if you take medicines for heart problems.
Exkivity and other medicines may affect each other causing serious side effects.
How should I take Exkivity?
- Take Exkivity exactly as your healthcare provider tells you to take it.
- Take your prescribed dose of Exkivity 1 time each day.
- Take Exkivity with or without food.
- Swallow Exkivity capsules whole. Do not open, chew, or dissolve the contents of the capsules.
- Do not change your dose or stop taking Exkivity unless your healthcare provider tells you to.
- Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Exkivity if you develop certain side effects.
- If you miss a dose of Exkivity, and it has been more than 6 hours, skip the dose and take your next dose at your regularly scheduled time the next day.
- If you vomit a dose of Exkivity, do not take an extra dose. Take your next dose at your regularly scheduled time the next day.
What should I avoid while taking Exkivity?
- Avoid eating grapefruit or drinking grapefruit juice during treatment with Exkivity. Grapefruit may increase the amount of Exkivity in your blood.
What are the possible side effects of Exkivity?
Exkivity may cause serious side effects, including:
See “What is the most important information I should know about Exkivity?”.
- Lung problems. Exkivity may cause severe lung problems that may lead to death. Symptoms may be similar to those symptoms from lung cancer. Tell your healthcare provider right away if you develop any new or worsening symptoms, including trouble breathing or shortness of breath, cough, chest pain, or fever.
- Heart problems, including heart failure. Exkivity may cause heart problems that may lead to death. Your healthcare provider should check your heart function before you start and during treatment with Exkivity. Tell your healthcare provider right away if you have any signs or symptoms of a heart problem, including feeling like your heart is pounding or racing, shortness of breath, chest pain, swelling of your ankles and feet, or feeling faint.
- Diarrhea. Diarrhea is common during treatment with Exkivity, and may sometimes be severe. Diarrhea can cause you to lose too much body fluid (dehydration) and kidney problems. Your healthcare provider may tell you to start drinking more fluids and electrolytes to replace body salts or start taking your antidiarrheal medicines. Tell your healthcare provider right away if you have any loose stools or have stools more often than is normal for you.
The most common side effects of Exkivity include:
- diarrhea
- rash
- nausea
- mouth sores
- vomiting
- decrease appetite
- infection of skin around nails
- tiredness
- dry skin
- muscle or bone pain
Exkivity may affect fertility in females and males, which may affect your ability to have a child. Talk to your healthcare provider if this is a concern for you.
These are not all of the possible side effects of Exkivity.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Exkivity
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Exkivity for a condition for which it was not prescribed. Do not give Exkivity to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Exkivity that is written for health professionals.
How should I store Exkivity?
- Store Exkivity at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Exkivity and all medicines out of the reach of children.
What are the ingredients in Exkivity?
Active ingredient: mobocertinib
Inactive ingredients: None
Capsule shells: gelatin and titanium dioxide. The printing ink contains shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, strong ammonia solution, black iron oxide, potassium hydroxide, and purified water.
Label
PRINCIPAL DISPLAY PANEL – 40 MG CAPSULE BOTTLE LABEL
- NDC 63020-040-12
- EXKIVITY™
mobocertinib - 40 mg capsules
- 120 capsules
- Rx Only
For Oral Use
Takeda

SRC: NLM .
