Equetro
Generic name: carbamazepine (oral)
Drug class: Dibenzazepine anticonvulsants
Medically reviewed by A Ras MD.
What is Equetro?
Equetro is a prescription medicine used to treat people with acute manic or mixed episodes that happen with Bipolar I Disorder certain types of nerve pain (trigeminal neuralgia and glossopharyngeal neuralgia) certain types of seizures (partial, tonic-clonic, mixed).
Equetro is not a regular pain medicine and should not be used for aches or pains.
Equetro should not be used to treat people with absence seizures (petit mal).
It is not known if Equetro is safe and effective in children and adolescents for the treatment of Bipolar I Disorder and nerve pain.
Description
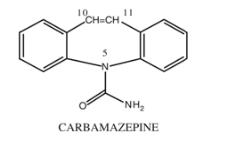
EQUETRO (carbamazepine) is a mood stabilizer available for oral administration as 100 mg, 200 mg, and 300 mg extended-release capsules of carbamazepine, USP. Carbamazepine is a white to off-white powder, practically insoluble in water and soluble in alcohol and in acetone. Its molecular weight is 236.27. The chemical name of carbamazepine is 5H-dibenz[b,f]azepine-5-carboxamide, and the structural formula is:
EQUETRO® is a multi-component capsule formulation consisting of three different types of beads: immediate-release beads, extended-release beads, and enteric-release beads. The three bead types are combined in a specific ratio to provide twice-daily dosing of EQUETRO®.
Inactive ingredients: citric acid, colloidal silicon dioxide, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, povidone, sodium lauryl sulfate, talc, triethyl citrate, and other ingredients.
The 100 mg capsule shells contain gelatin-NF, FD&C Blue #2, Yellow Iron Oxide, and Titanium Dioxide, and are imprinted with white ink; the 200 mg capsule shells contain gelatin-NF, Yellow Iron Oxide, FD&C Blue #2, and Titanium Dioxide, and are imprinted with white ink; and the 300 mg capsule shells contain gelatin-NF, FD&C Blue #2, Yellow Iron Oxide, and Titanium Dioxide, and are imprinted with white ink.
Mechanism of Action
Although numerous pharmacological effects of carbamazepine have been described in the published literature (e.g., modulation of ion channels [sodium and calcium], receptor-mediated neurotransmission [GABAergic, glutamatergic, and monoaminergic], and intracellular signaling pathways in experimental preparations), the contribution of these effects to the efficacy of carbamazepine in acute manic or mixed episodes associated with bipolar disorder is unknown.
What is the most important information I should know about Equetro?
Do not stop taking Equetro without first talking to your healthcare provider.
Stopping Equetro suddenly can cause serious problems.
If you have any of the problems listed below call your healthcare provider right away.
Equetro can cause serious side effects, including:
1. Equetro may cause rare but serious rashes that may lead to death. These serious skin reactions are more likely to happen within the first four months after you start taking Equetro, but may occur at later times. These reactions can happen to anyone, but are more likely in people of Asian descent. If you are of Asian descent, you may need a genetic blood test before you take Equetro to see if you are at higher risk for serious skin reactions with this medicine. Symptoms may include:
- skin rash
- hives
- sores in your mouth
- blistering or peeling of the skin
2. Equetro may cause rare but serious blood problems. Symptoms may include:
- fever
- shortness of breath
- fatigue
- easy bruising
- red or purple spots on your body
- unusual bleeding such as bleeding gums, nose bleeds, or heavy menstrual bleeding
- swollen glands and sore throat
3. Equetro may cause a serious or life-threatening allergic reaction that may affect your skin or other parts of your body such as your liver or blood cells. You may or may not have rash with these types of reactions. Call a healthcare provider right away if you have any of the following symptoms:
- skin rash
- hives
- fever
- swollen glands that do not go away
- swelling of your lips or tongue
- yellowing of your skin or eyes
- unusual bruising or bleeding
- severe fatigue or weakness
- unexpected, severe muscle pain
- frequent infections
These symptoms may be the first signs of a serious reaction. A healthcare provider should examine you to decide if you should continue taking Equetro.
4. Equetro may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call your healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity or talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop Equetro without first talking to a healthcare provider.
Stopping Equetro suddenly can cause serious problems. You should talk to your healthcare provider before stopping.
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
Who should not take Equetro?
Do not take Equetro if you:
- have a history of bone marrow depression.
- are allergic to carbamazepine or any of the ingredients in Equetro. See the end of this Medication Guide for a complete list of ingredients in Equetro.
- are allergic to medicines called tricyclic antidepressants (TCAs). Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
- are taking delaviridine or other medicines called reverse transcriptase inhibitors. Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
- have taken a medicine called a Monoamine Oxidase Inhibitor (MAOI) in the last 14 days. Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
- are taking nefazodone.
What should I tell my healthcare provider before taking Equetro?
Before you take Equetro, tell your healthcare provider if you:
- have or have had suicidal thoughts or actions, depression or mood problems
- have or ever had heart problems
- have or ever had blood problems
- have or ever had liver problems
- have or ever had kidney problems
- have or ever had allergic reactions to medicines
- have or ever had increased pressure in your eye
- have any other medical conditions
- drink grapefruit juice or eat grapefruit
- use birth control. Equetro may make your birth control less effective. Tell your healthcare provider if your menstrual bleeding changes while you take birth control and Equetro.
- are pregnant or plan to become pregnant. Equetro may harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking Equetro. You and your healthcare provider will decide if you should take Equetro while you are pregnant.
- If you become pregnant while taking Equetro, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic medicines during pregnancy. Equetro is also an antiepileptic drug. You can enroll in this registry by calling 1-888-233-2334.
- are breastfeeding or plan to breastfeed. Equetro can pass into breast milk. You and your healthcare provider should decide if you will take Equetro or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Taking Equetro with certain other medicines may cause side effects or affect how well they work. Do not stop or start other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take Equetro?
- Do not stop taking Equetro without first talking to your healthcare provider. Stopping Equetro suddenly can cause serious problems.
- Take Equetro exactly as prescribed. Your healthcare provider will tell you how much Equetro to take.
- Your healthcare provider may change your dose. Do not change your dose of Equetro without talking to your healthcare provider.
- Swallow Equetro capsules whole. Do not crush or chew.
- If you cannot swallow Equetro capsules whole, you may open the Equetro capsules and sprinkle the beads over food such as a teaspoon of applesauce and swallow the mixture. Do not crush or chew the beads.
- Take Equetro with or without food.
- If you take too much Equetro, call your healthcare provider or local Poison Control Center right away.
What should I avoid while taking Equetro?
- Do not drink alcohol or take other drugs that make you sleepy or dizzy while taking Equetro until you talk to your healthcare provider. Equetro taken with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how Equetro affects you. Equetro can slow your thinking and motor skills.
What are the possible side effects of Equetro?
See “What is the most important information I should know about Equetro?”
Equetro may cause other serious side effects, including:
- Seizure risk. Stopping Equetro suddenly may cause you to have a seizure. The risk of seizures may be higher in people who already have seizures. Do not stop taking Equetro without first talking with your healthcare provider.
- Low level of sodium in your blood (hyponatremia). Symptoms of hyponatremia may include:
- headache
- new seizures or an increased number of seizures
- difficulty concentrating
- memory problems
- confusion
- weakness
- balance problems
- Problems with judgment, thinking, and movement.
- Liver problems. Symptoms of liver problems may include:
Get medical help right away if you have any of the symptoms listed above or listed in “What is the most important information I should know about Equetro?”
The most common side effects of Equetro include:
- dizziness
- drowsiness
- nausea
- vomiting
- problems with walking and coordination (unsteadiness)
- constipation
- itching
- dry mouth
- weakness
- blurred vision
- problems speaking
These are not all the possible side effects of Equetro. For more information, ask your healthcare provider or pharmacist.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
To report suspected adverse reactions, contact Validus Pharmaceuticals LLC at 1-866-9VALIDUS(1-866-982-5438) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
General information about the safe and effective use of Equetro
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Equetro for a condition for which it was not prescribed. Do not give Equetro to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Equetro. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Equetro that is written for health professionals.
For more information, go to www.EQUETRO.com or call 1-866-982-5438.
How should I store Equetro?
- Store Equetro between 680F to 770F (200C to 250C).
- Keep Equetro capsules out of the light.
- Keep Equetro capsules dry.
Keep Equetro and all medicines out of the reach of children.
What are the ingredients in Equetro?
Active ingredient: carbamazepine
Inactive ingredients: citric acid, colloidal silicon dioxide, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, povidone, sodium lauryl sulfate, talc, triethyl citrate, and other ingredients.
- 100mg capsule shell contains: gelatin-NF, FD&C Blue #2, Yellow Iron Oxide, and Titanium Dioxide, and are imprinted with white ink.
- 200mg capsule shell contains: gelatin-NF, Yellow Iron Oxide, FD&C Blue #2, and Titanium Dioxide, and are imprinted with white ink.
- 300mg capsule shell contains: gelatin-NF, FD&C Blue #2, Yellow Iron Oxide, and Titanium Dioxide, and are imprinted with white ink.
Label
PRINCIPAL DISPLAY PANEL
- NDC 30698-419-12
Equetro® Extended – Release Capsules - (carbamazepine)
100 mg
120 Capsules
Rx Only
PRINCIPAL DISPLAY PANEL
- NDC 30698-421-12
Equetro® Extended – Release Capsules
(carbamazepine) - 200 mg
120 Capsules
Rx Only

PRINCIPAL DISPLAY PANEL
- NDC 30698-423-12
Equetro® Extended – Release Capsules
(carbamazepine) - 300 mg
120 Capsules
Rx Only

SRC: NLM .
