Eprontia
Generic name: topiramate
Dosage form: oral solution
Drug class: Carbonic anhydrase inhibitor anticonvulsants
Medically reviewed by A Ras MD.
What is Eprontia?
Eprontia is a prescription medicine used to treat certain types of seizures (partial-onset seizures and primary generalized tonic-clonic seizures) in adults and children 2 years and older, with other medicines to treat certain types of seizures (partial-onset seizures, primary generalized tonic-clonic seizures, and seizures associated with Lennox-Gastaut syndrome) in adults and children 2 years and older, to prevent migraine headaches in adults and adolescents 12 years and older.
Description
EPRONTIA (topiramate) oral solution is available as a 25 mg/mL solution for oral administration.
Topiramate has the molecular formula C12H21NO8S and a molecular weight of 339.36. Topiramate is designated chemically as 2,3:4,5-Di-O-isopropylidene-β-D-fructopyranose sulfamate and has the following structural formula:
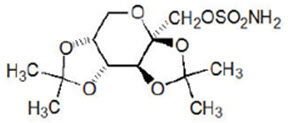
Topiramate is a white crystalline powder with a bitter taste. Topiramate is a sulfamate-substituted monosaccharide. Topiramate is most soluble in alkaline solutions containing sodium hydroxide or sodium phosphate and having a pH of 9 to 10. It is freely soluble in acetone, chloroform, dimethylsulfoxide, and ethanol. The solubility in water is 9.8 mg/mL. Its saturated solution has a pH of 6.3.
EPRONTIA oral solution is colorless to slightly yellow colored clear viscous liquid. EPRONTIA contains the following inactive ingredients: glycerin, methylparaben, mixed berry flavor, polyethylene glycol, propylparaben, and sucralose.
Mechanism of Action
The precise mechanisms by which topiramate exerts its anticonvulsant and preventive migraine effects are unknown; however, preclinical studies have revealed four properties that may contribute to topiramate’s efficacy for epilepsy and the preventive treatment of migraine. Electrophysiological and biochemical evidence suggests that topiramate, at pharmacologically relevant concentrations, blocks voltage-dependent sodium channels, augments the activity of the neurotransmitter gamma-aminobutyrate at some subtypes of the GABA-A receptor, antagonizes the AMPA/kainate subtype of the glutamate receptor, and inhibits the carbonic anhydrase enzyme, particularly isozymes II and IV.
What is the most important information I should know about Eprontia?
Eprontia may cause eye problems. Serious eye problems include:
- any sudden decrease in vision with or without eye pain and redness.
- a blockage of fluid in the eye causing increased pressure in the eye (secondary angle closure glaucoma).
- These eye problems can lead to permanent loss of vision if not treated.
- You should call your healthcare provider right away if you have any new eye symptoms, including any new problems with your vision.
Eprontia may cause decreased sweating and increased body temperature (fever). People, especially children, should be watched for signs of decreased sweating and fever, especially in hot temperatures. Some people may need to be hospitalized for this condition. If a high fever, a fever that does not go away, or decreased sweating develops, call your healthcare provider right away.
Eprontia can increase the level of acid in your blood (metabolic acidosis). If left untreated, metabolic acidosis can cause brittle or soft bones (osteoporosis, osteomalacia, osteopenia), kidney stones, can slow the rate of growth in children, and may possibly harm your baby if you are pregnant. Metabolic acidosis can happen with or without symptoms. Sometimes people with metabolic acidosis will:
- feel tired
- not feel hungry (loss of appetite)
- feel changes in heartbeat
- have trouble thinking clearly
Your healthcare provider should do a blood test to measure the level of acid in your blood before and during your treatment with Eprontia. If you are pregnant, you should talk to your healthcare provider about whether you have metabolic acidosis.
Like other antiepileptic drugs, Eprontia may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- new or worse depression
- feeling agitated or restless
- trouble sleeping (insomnia)
- acting aggressive, being angry, or violent
- an extreme increase in activity and talking (mania)
- attempts to commit suicide
- new or worse anxiety
- panic attacks
- new or worse irritability
- acting on dangerous impulses
- other unusual changes in behavior or mood
Do not stop Eprontia without first talking to a healthcare provider.
- Stopping Eprontia suddenly can cause serious problems.
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Eprontia can harm your unborn baby.
- If you take Eprontia during pregnancy, your baby has a higher risk for birth defects called cleft lip and cleft palate. These defects can begin early in pregnancy, even before you know you are pregnant.
- Cleft lip and cleft palate may happen even in children born to women who are not taking any medicines and do not have other risk factors.
- There may be other medicines to treat your condition that have a lower chance of birth defects.
- All women of childbearing age should talk to their healthcare providers about using other possible treatments instead of Eprontia. If the decision is made to use Eprontia, you should use effective birth control (contraception) unless you are planning to become pregnant. You should talk to your healthcare provider about the best kind of birth control to use while you are taking Eprontia.
- Tell your healthcare provider right away if you become pregnant while taking Eprontia. You and your healthcare provider should decide if you will continue to take Eprontia while you are pregnant.
- If you take Eprontia during pregnancy, your baby may be smaller than expected at birth. The long-term effects of this are not known. Talk to your healthcare provider if you have questions about this risk during pregnancy.
- Metabolic acidosis may have harmful effects on your baby. Talk to your healthcare provider if Eprontia has caused metabolic acidosis during your pregnancy.
Pregnancy Registry: If you become pregnant while taking Eprontia, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of Eprontia and other antiepileptic drugs during pregnancy.
What should I tell my healthcare provider before taking Eprontia?
Before taking Eprontia, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had depression, mood problems, or suicidal thoughts or behavior.
- have kidney problems, have kidney stones, or are getting kidney dialysis.
- have a history of metabolic acidosis (too much acid in the blood).
- have liver problems.
- have weak, brittle, or soft bones (osteomalacia, osteoporosis, osteopenia, or decreased bone density).
- have lung or breathing problems.
- have eye problems, especially glaucoma.
- have diarrhea.
- have a growth problem.
- are on a diet high in fat and low in carbohydrates, which is called a ketogenic diet.
- are having surgery.
- are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. Eprontia passes into breast milk. Breastfed babies may be sleepy or have diarrhea. It is not known if the Eprontia that passes into breast milk can cause other serious harm to your baby. Talk to your healthcare provider about the best way to feed your baby if you take Eprontia.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Eprontia and other medicines may affect each other causing side effects.
Especially tell your healthcare provider if you take:
- Valproic acid (such as Depakote).
- any medicines that impair or decrease your thinking, concentration, or muscle coordination.
- birth control pills. Eprontia may make your birth control pills less effective. Tell your healthcare provider if your menstrual bleeding changes while you are taking birth control pills and Eprontia.
Ask your healthcare provider if you are not sure if your medicine is listed above.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine. Do not start a new medicine without talking with your healthcare provider.
How should I take Eprontia?
- Take Eprontia Oral Solution exactly as prescribed.
- Your healthcare provider may change your dose. Do not change your dose without talking to your healthcare provider.
- Eprontia can be taken with or without food. Drink plenty of fluids during the day. This may help prevent kidney stones while taking Eprontia.
- Take Eprontia using a marked (calibrated) measuring device. Ask your pharmacist to recommend a measuring device and for instructions on how to measure the correct dose. Do not use a household teaspoon or tablespoon.
- If you take too much Eprontia, call your healthcare provider right away or go to the nearest hospital emergency room right away.
- If you miss a single dose of Eprontia, take it as soon as you can. However, if you are within 6 hours of taking your next scheduled dose, wait until then to take your usual dose of Eprontia, and skip the missed dose. Do not double your dose. If you have missed more than 1 dose, you should call your healthcare provider for advice.
- Do not stop taking Eprontia without talking to your healthcare provider. Stopping Eprontia suddenly may cause serious problems. If you have epilepsy and you stop taking Eprontia suddenly, you may have seizures that do not stop. Your healthcare provider will tell you how to stop taking Eprontia slowly.
Your healthcare provider may do blood tests while you take Eprontia.
What should I avoid while taking Eprontia?
- you should not drink alcohol while taking Eprontia. Eprontia and alcohol can affect each other causing side effects such as sleepiness and dizziness.
- Do not drive a car or operate machinery until you know how Eprontia affects you. Eprontia can slow your thinking and motor skills and may affect vision
What are the possible side effects of Eprontia?
See “What is the most important information I should know about Eprontia?”
- High blood ammonia levels. High ammonia in the blood can affect your mental activities, slow your alertness, make you feel tired, or cause vomiting. This has happened when Eprontia is taken with a medicine called valproic acid (Depakote). Call your healthcare provider right away if you develop unexplained tiredness, vomiting, slowing of your thinking or reaction time, or changes in your mental activities.
- Effects on thinking and alertness. Eprontia may affect how you think and cause confusion, problems with concentration, attention, memory, or speech. Eprontia may cause depression or mood problems, tiredness, and sleepiness.
- Dizziness or loss of muscle coordination.
- Serious skin reactions. Eprontia may cause a severe rash with blisters and peeling skin, especially around the mouth, nose, eyes, and genitals (Stevens-Johnson syndrome). Eprontia may also cause a rash with blisters and peeling skin over much of the body that may cause death (toxic epidermal necrolysis). Call your healthcare provider right away if you develop a skin rash or blisters.
- Kidney stones. Drink plenty of fluids when taking Eprontia to decrease your chances of getting kidney stones.
- Low body temperature. Taking Eprontia when you are also taking valproic acid can cause a drop in body temperature to less than 95°F, or can cause tiredness, confusion, or coma.
Call your healthcare provider right away if you have any of the symptoms above.
The most common side effects of Eprontia include:
- tingling of the arms and legs nervousness slow reactions (paresthesia)
- nausea
- diarrhea
- nervousness
- speech problems
- dizziness
- slow reactions
- pain in the abdomen
- decreased feeling or sensitivity, especially in the skin
- not feeling hungry
- a change in the way foods taste
- weight loss
- upper respiratory tract infection
- tiredness
- sleepiness/drowsiness
- difficulty with memory
- fever
- abnormal vision
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of Eprontia. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1- 800-FDA-1088. You may also report side effects to Azurity Pharmaceuticals, Inc. at 1-855-379-0383.
General information about the safe and effective use of Eprontia
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Eprontia for a condition for which it was not prescribed. Do not give Eprontia to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Eprontia that is written for health professionals.
How should I store Eprontia?
- Store Eprontia at room temperature between 68°F to 77°F (20°C to 25°C).
- Throw away (discard) unused portion 30 days after first opening.
- Avoid freezing and excessive heat.
- Keep container tightly closed
Keep Eprontia and all medicines out of the reach of children.
What are the ingredients in Eprontia?
Active ingredient: Topiramate
Inactive ingredients: glycerin, methylparaben, mixed berry flavor, polyethylene glycol, propylparaben, and sucralose.
Label
PRINCIPAL DISPLAY PANEL – BOTTLE LABEL
- NDC 52652-9001-1 Rx Only
- EPRONTIA™
(topiramate)
oral solution - 25 mg/mL
- FOR ORAL USE ONLY
- ATTENTION PHARMACIST:
- Dispense with the following to each patient:

SRC: NLM .
