Envarsus XR
Generic name: Tacrolimus Extended-Release Tablets
Drug class: Calcineurin inhibitors
Medically reviewed by A Ras MD.
What is Envarsus XR?
Envarsus XR is a prescription medicine used with other medicines to help prevent organ rejection in people who have had a kidney transplant.
Envarsus XR is an extended-release tablet and is not the same as tacrolimus extended-release capsules, tacrolimus [immediate-release] capsules or tacrolimus for oral suspension. Your healthcare provider should decide what medicine is right for you.
Description
Tacrolimus is the active ingredient in ENVARSUS XR. Tacrolimus is a calcineurin-inhibitor immunosuppressant produced by Streptomyces tsukubaensis. Chemically, tacrolimus is designated as [3S-[3R*[E(1S*,3S*,4S*)],4S*,5R*,8S*,9E,12R*,14R*,15S*,16R*,18S*,19S*,26aR*]]-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclo-hexyl)-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-15,19-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, monohydrate.
The chemical structure of tacrolimus is:
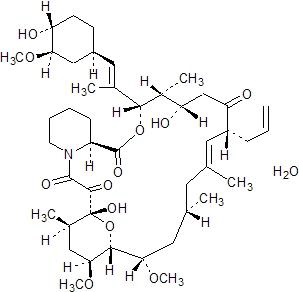
Tacrolimus has an empirical formula of C44H69NO12•H2O and a formula weight of 822.03. Tacrolimus appears as white crystals or crystalline powder. It is practically insoluble in water, freely soluble in ethanol, and very soluble in methanol and chloroform.
ENVARSUS XR is available for oral administration as extended-release tablets containing the equivalent of 0.75 mg, 1 mg, or 4 mg of anhydrous tacrolimus USP. Inactive ingredients include hypromellose USP, lactose monohydrate NF, polyethylene glycol NF, poloxamer NF, magnesium stearate NF, tartaric acid NF, butylated hydroxytoluene NF, and dimethicone NF.
Mechanism of Action
Tacrolimus binds to an intracellular protein, FKBP-12. A complex of tacrolimus-FKBP-12, calcium, calmodulin, and calcineurin (an ubiquitous mammalian intracellular enzyme) is then formed and the phosphatase activity of calcineurin inhibited. Such inhibition prevents the dephosphorylation and translocation of various factors such as the nuclear factor of activated T-cells (NF-AT) and nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB).
Tacrolimus inhibits the expression and/or production of several cytokines that include interleukin (IL)-1 beta, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, gamma interferon, tumor necrosis factor-alpha, and granulocyte macrophage colony stimulating factor. Tacrolimus also inhibits IL-2 receptor expression and nitric oxide release, induces apoptosis and production of transforming growth factor-beta that can lead to immunosuppressive activity. The net result is the inhibition of T-lymphocyte activation and proliferation as well as T-helper-cell-dependent B-cell response (i.e., immunosuppression).
What is the most important information I should know about Envarsus XR?
Envarsus XR can cause serious side effects, including:
- Increased risk of cancer. People who take Envarsus XR have an increased risk of getting some kinds of cancer, including skin and lymph gland cancer (lymphoma).
- Increased risk of infection. Envarsus XR is a medicine that affects your immune system. Envarsus XR can lower the ability of your immune system to fight infections. Serious infections can happen in people receiving Envarsus XR that can cause death.
Call your doctor right away if you have symptoms of an infection such as:- fever
- cough or flu-like symptoms
- warm, red, or painful areas on your skin
- muscle aches
- sweats or chills
Who should not take Envarsus XR?
Do not take Envarsus XR if you are allergic to tacrolimus or any of the ingredients in Envarsus XR. See the end of this leaflet for a complete list of ingredients in Envarsus XR.
What should I tell my healthcare provider before taking Envarsus XR?
Before you take Envarsus XR, tell your healthcare provider if you:
- plan to receive any live vaccines. Ask your healthcare provider if you are not sure if your vaccine is a live vaccine.
- have or have had liver, kidney or heart problems.
- have any other medical conditions.
- are pregnant or plan to become pregnant. Envarsus XR may harm your unborn baby.
- If you are able to become pregnant, you should use effective birth control before and during treatment with Envarsus XR. Talk to your healthcare provider about birth control methods that may be right for you.
- Males who have female partners that are able to become pregnant should also use effective birth control before and during treatment with Envarsus XR. Talk to your healthcare provider before starting treatment with Envarsus XR about birth control methods that may be right for you.
- There is a pregnancy registry for females who become pregnant and males who have fathered a pregnancy during treatment with Envarsus XR. The purpose of this registry is to collect information about your health and of your baby. To enrol in this voluntary registry, call 1-877- 955-6877.
- are breastfeeding or plan to breastfeed. Envarsus XR passes into your breast milk. You and your healthcare provider should decide if you will breastfeed while taking Envarsus XR
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, natural, herbal or nutritional supplements.
Envarsus XR may affect the way other medicines work, and other medicines may affect how Envarsus XR works.
How should I take Envarsus XR?
- Take Envarsus XR exactly as your healthcare provider tells you to take it.
- Your healthcare provider may change your dose of Envarsus XR if needed. Do not stop taking or change your dose of Envarsus XR without talking to your healthcare provider.
- Take Envarsus XR once daily with fluid (preferably water) on an empty stomach, at least 1 hour before or at least 2 hours after a meal, at the same time each day (preferably in the morning).
- Take Envarsus XR tablets whole. Do not chew, divide, crush, or dissolve Envarsus XR tablets before swallowing. If you cannot swallow Envarsus XR tablets whole, tell your healthcare provider.
- If you miss your dose of Envarsus XR, it should be taken as soon as possible, but no longer than 15 hours after missing your dose. If the time after missing your dose is more than 15 hours, the missed dose should be skipped and the next dose should be taken the following morning at your regularly scheduled time. Do not take 2 doses at the same time.
- If you take too much Envarsus XR, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking Envarsus XR?
- Live vaccines such as flu vaccine through your nose, measles, mumps, rubella, polio by mouth, BCG (TB vaccine), yellow fever, chicken pox (varicella), or typhoid.
- Exposure to sunlight and UV light such as tanning machines. Wear protective clothing and use a sunscreen.
- You should not eat grapefruit or drink grapefruit juice while taking Envarsus XR.
- You should not drink alcohol while taking Envarsus XR.
What are the possible side effects of Envarsus XR?
Envarsus XR may cause serious side effects, including:
- See “What the most important information I should know about Envarsus XR?”
- problems from medication errors such as graft rejection and other serious reactions. People who take Envarsus XR have sometimes been given the wrong medicine because some medicines have the same ingredient (tacrolimus) as Envarsus XR. Check your Envarsus XR when you get a new prescription to make sure you have received the right medicine.
- Call your healthcare provider right away if you think you were given the wrong medicine.
- Ask your healthcare provider or pharmacist if you are not sure what Envarsus XR should look like.
- high blood sugar (diabetes). Your healthcare provider may do certain tests to check for diabetes while you take Envarsus XR. Call your healthcare provider right away if you have:
- kidney problems. Kidney problems are serious and common side effects of Envarsus XR. Your healthcare provider may do certain tests to check for kidney function while you take Envarsus XR.
- nervous system problems. Nervous system problems are a serious and common side effect of Envarsus XR. Call your healthcare provider right away if you get any of these symptoms while taking Envarsus XR. These could be signs of serious nervous system problems:
- confusion
- coma
- seizures
- numbness and tingling
- headache
- vision changes
- muscle tremors
- high levels of potassium in your blood. Your healthcare provider may do certain tests to check your potassium level while you take Envarsus XR.
- high blood pressure. Your healthcare provider will monitor your blood pressure while you take Envarsus XR.
- changes in the electrical activity of your heart (QT prolongation).
- severe low blood cell count (anemia)
The most common side effects of Envarsus XR are diarrhea, urinary tract infection, low red blood cell count (anemia), high blood pressure, and constipation.
These are not all the possible side effects of Envarsus XR. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Envarsus XR
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Envarsus XR for a condition for which it was not prescribed. Do not give Envarsus XR to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Envarsus XR. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Envarsus XR that is written for health professionals. For more information, go to www.ENVARSUSXR.com or call 1-844-Veloxis (1-844-835-6947).
How should I store Envarsus XR?
- Store Envarsus XR at room temperature between 68 ºF to 77 ºF (20 °C to 25 ºC).
- Safely throw away medicine that is out of date or no longer needed.
Keep Envarsus XR and all medicines out of reach of children.
What are the ingredients in Envarsus XR?
Active ingredient: tacrolimus USP
Inactive ingredients: hypromellose USP, lactose monohydrate NF, polyethylene glycol NF, poloxamer NF, magnesium stearate NF, tartaric acid NF, butylated hydroxytoluene NF, and dimethicone NF
Label
PRINCIPAL DISPLAY PANEL – Envarsus 0.75 mg 30 Count Carton Label
- NDC 68992-3075-3
- Envarsus XR®
- (tacrolimus
extended-release tablets) - 0.75 mg
- ONCE-DAILY
- Swallow tablet whole.
- Do not chew, divide,
or crush tablet. - Dosage: See Package Insert
for dosage information. - Always dispense with a
Medication Guide - For oral use only
- Veloxis PHARMACEUTICALS
- 30 Tablets
PRINCIPAL DISPLAY PANEL – Envarsus 1 mg 30 Count Carton Label
- NDC 68992-3010-3
- Envarsus XR®
- (tacrolimus
extended-release tablets) - 1 mg
- ONCE-DAILY
- Swallow tablet whole.
- Do not chew, divide,
or crush tablet. - Dosage: See Package Insert
for dosage information. - Always dispense with a
Medication Guide - For oral use only
- Veloxis PHARMACEUTICALS
- 30 Tablets

PRINCIPAL DISPLAY PANEL – Envarsus 4 mg 30 Count Carton Label
- NDC 68992-3040-3
- Envarsus XR®
- (tacrolimus
extended-release tablets) - 4 mg
- ONCE-DAILY
- Swallow tablet whole.
- Do not chew, divide,
or crush tablet. - Dosage: See Package Insert
for dosage information. - Always dispense with a
Medication Guide - For oral use only
- Veloxis PHARMACEUTICALS
- 30 Tablets
SRC: NLM .
