Entocort EC
Generic name: budesonide (oral)
Brand names: Entocort EC, Ortikos, Uceris
Drug class: Glucocorticoids
Medically reviewed by A Ras MD.
What is Entocort EC?
Entocort EC is a prescription corticosteroid medicine used to treat mild to moderate Crohn’s disease that affects part of the small intestine (ileum) and part of the large intestine (ascending colon) in people 8 years of age and older with active Crohn’s disease, in adults to help keep symptoms from coming back for up to 3 months.
It is not known if Entocort EC is safe and effective in children under 8 years of age, or in children 8 to 17 years of age who weigh 55 pounds (25 kg) or less, for the treatment of mild to moderate active Crohn’s disease that affects part of the small intestine (ileum) and part of the large intestine (ascending colon).
It is not known if Entocort EC is safe and effective in children to help keep symptoms of mild to moderate Crohn’s disease that affects part of the small intestine (ileum) and part of the large intestine (ascending colon) from coming back.
Description
Budesonide, the active ingredient of ENTOCORT® EC capsules, is a synthetic corticosteroid. It is designated chemically as (RS)-11β, 16α, 17,21-tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde. Budesonide is provided as a mixture of two epimers (22R and 22S). The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5. Its structural formula is:
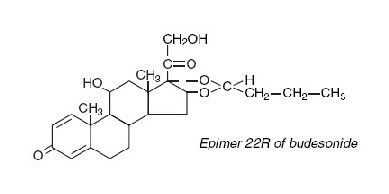
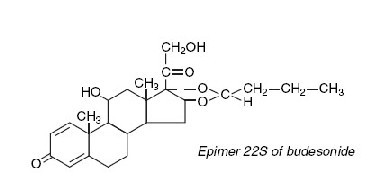
Budesonide is a white to off-white, tasteless, odorless powder that is practically insoluble in water and heptane, sparingly soluble in ethanol, and freely soluble in chloroform. Its partition coefficient between octanol and water at pH 5 is 1.6 x 103 ionic strength 0.01.
Each capsule contains 3 mg of micronized budesonide with the following inactive ingredients: ethylcellulose, acetyltributyl citrate, methacrylic acid copolymer type C, triethyl citrate, antifoam M, polysorbate 80, talc, and sugar spheres. The capsule shells have the following inactive ingredients: gelatin, iron oxide, and titanium dioxide.
Who should not take Entocort EC?
Do not take Entocort EC if:
- you are allergic to budesonide or any of the ingredients in Entocort EC. See the end of this leaflet for a complete list of ingredients in Entocort EC.
What should I tell my healthcare provider before taking Entocort EC?
Before you take Entocort EC tell your healthcare provider if you have any other medical conditions including if you:
- have liver problems.
- are planning to have surgery.
- have chicken pox or measles or have recently been near anyone with chicken pox or measles.
- have an infection.
- have diabetes or glaucoma or have a family history of diabetes or glaucoma.
- have cataracts.
- have or had tuberculosis.
- have high blood pressure (hypertension).
- have decreased bone mineral density (osteoporosis).
- have stomach ulcers.
- are pregnant or plan to become pregnant. Entocort EC may harm your unborn baby. Talk to your healthcare provider about the possible risk to your unborn baby if you take Entocort EC when you are pregnant. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during your treatment with Entocort EC.
- are breastfeeding or plan to breastfeed. It is not known if Entocort EC passes into your breast milk or if it will affect your baby. Talk to your healthcare provider about the best way to feed your baby if you take Entocort EC.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Entocort EC and other medicines may affect each other causing side effects.
How should I take Entocort EC?
- Take Entocort EC exactly as your healthcare provider tells you.
- Your healthcare provider will tell you how many Entocort EC capsules to take. Your healthcare provider may change your dose if needed.
- Take Entocort EC 1 time each day in the morning.
- Take Entocort EC capsules whole. Do not chew or crush Entocort EC capsules before swallowing.
- For patients unable to swallow a whole capsule, Entocort EC capsules can be opened and administered as follows:
- 1. Place 1 tablespoonful of applesauce into a clean container, such as an empty bowl. The applesauce used should not be hot and should be soft enough to be swallowed without chewing.
- 2. Open the capsule. You may need to use more than 1 Entocort EC capsule for the dose prescribed by your healthcare provider.
- 3. Carefully empty all of the granules inside the capsule on the applesauce.
- 4. Stir the granules with the applesauce.
- 5. Swallow the applesauce and granules mixture within 30 minutes after preparing it. Follow the applesauce and granules immediately with a glass (8 ounces) of cool water to help with complete swallowing of the granules.
- 6. Do not chew or crush the granules.
- 7. Do not save the applesauce and granules for later use.
- If you take too much Entocort EC call your healthcare provider right away or go to the nearest hospital emergency room.
What should I avoid while taking Entocort EC?
- Do not drink grapefruit juice during your treatment with Entocort EC. Drinking grapefruit juice can increase the level of Entocort EC in your blood.
What are the possible side effects of Entocort EC?
Entocort EC may cause serious side effects, including:
- Effects of having too much corticosteroid medicine in your blood (hypercorticism). Long-time use of Entocort EC can cause you to have too much corticosteroid medicine in your blood. Tell your healthcare provider if you have any of the following signs and symptoms of hypercorticism:
- acne
- thicker or more hair on your body and face
- bruise easily
- a fatty pad or hump between your shoulders (buffalo hump)
- rounding of your face (moon face)
- pink or purple stretch marks on the skin of your abdomen, thighs, breasts and arms
- ankle swelling
- Adrenal suppression. When Entocort EC is taken for a long period of time (chronic use), adrenal suppression can happen. This is a condition in which the adrenal glands do not make enough steroid hormones. Symptoms of adrenal suppression include: tiredness, weakness, nausea and vomiting and low blood pressure. Tell your healthcare provider if you are under stress or have any symptoms of adrenal suppression during treatment with Entocort EC.
- Worsening of allergies. If you take certain other corticosteroid medicines to treat allergies, switching to Entocort EC may cause your allergies to come back. These allergies may include a skin condition called eczema or inflammation inside your nose (rhinitis). Tell your healthcare provider if any of your allergies become worse while taking Entocort EC.
- Increased risk of infection. Entocort EC weakens your immune system. Taking medicines that weaken your immune system makes you more likely to get infections. Avoid contact with people who have contagious diseases, such as chicken pox or measles, while taking Entocort EC. Tell your healthcare provider right away if you come in contact with anyone who has chicken pox or measles.
- Tell your healthcare provider about any signs or symptoms of infection during treatment with Entocort EC, including:
- fever
- chills
- pain
- feeling tired
- aches
- nausea and vomiting
The most common side effects of Entocort EC in adults include:
- headache
- vomiting
- stomach area (abdominal) pain
- back pain
- infection in your air passages (respiratory infection)
- tiredness
- gas
- indigestion
- nausea
- pain
- dizziness
The most common side effects of Entocort EC in children 8 to 17 years of age, who weigh more than 55 pounds (25 kg), are similar to the most common side effects in adults.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Entocort EC. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Entocort EC
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide or Patient Information leaflet. Do not use Entocort EC for a condition for which it was not prescribed. Do not give Entocort EC to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Entocort EC that is written for health professionals.
How should I store Entocort EC?
- Store Entocort EC at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Entocort EC in a tightly closed container.
Keep Entocort EC and all medicines out of the reach of children.
What are the ingredients in Entocort EC?
Active ingredient: budesonide
Inactive ingredients: ethylcellulose, acetyltributyl citrate, methacrylic acid copolymer type C, triethyl citrate, antifoam M, polysorbate 80, talc, and sugar spheres.
The capsule shell contains: gelatin, iron oxide, and titanium dioxide.
Label
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

SRC: NLM .
