Enspryng
Generic name: satralizumab
Drug class: Interleukin inhibitors
Medically reviewed by A Ras MD.
What is Enspryng?
Enspryng is a prescription medicine used to treat neuromyelitis optica spectrum disorder (NMOSD) in adults who are aquaporin-4 (AQP4) antibody positive.
It is not known if Enspryng is safe and effective in children.
Description
Satralizumab-mwge is a recombinant humanized anti-human interleukin 6 (IL-6) receptor monoclonal antibody based on a human IgG2 framework. Each light chain and heavy chain consists of 214 and 443 amino acids, respectively. Satralizumab-mwge is a glycoprotein with an approximate molecular weight of 143 kDa and is produced by recombinant DNA technology in Chinese hamster ovary cells. The binding of satralizumab-mwge to the IL-6 receptor is pH-sensitive.
ENSPRYNG (satralizumab-mwge) injection for subcutaneous administration is supplied as a sterile, clear, colorless to slightly yellow solution with no preservative with an approximate pH of 6. ENSPRYNG is supplied in a single-dose prefilled syringe. Each syringe delivers 1 mL of solution containing 120 mg of satralizumab-mwge, L-arginine (26.1 mg), L-histidine (3.1 mg), poloxamer 188 (0.5 mg), L-aspartic acid (pH adjustment), and Water for Injection, USP.
Mechanism of Action
The precise mechanism by which satralizumab-mwge exerts therapeutic effects in NMOSD is unknown but is presumed to involve inhibition of IL-6-mediated signaling through binding to soluble and membrane-bound IL-6 receptors.
What is the most important information I should know about Enspryng?
1. Infections. Enspryng can increase your risk of serious infections some of which can be life-threatening. Talk to your healthcare provider if you are being treated for an infection or call them right away if you think you have signs of an infection, with or without a fever, such as:
- chills, feeling tired, muscle aches, cough that will not go away or a sore throat
- skin redness, swelling, tenderness, pain or sores on your body
- diarrhea, belly pain, or feeling sick
- burning when you urinate or urinating more often than usual
Your healthcare provider will check if you have an infection and treat it if needed before you start or continue to take Enspryng.
- Your healthcare provider should test you for hepatitis and tuberculosis (TB) before you start taking Enspryng.
- All required vaccinations should be completed before starting Enspryng. People using Enspryng should not be given ‘live’ or ‘live-attenuated’ vaccines. ‘Live’ or ‘live-attenuated’ vaccines should be given at least 4 weeks before you start Enspryng. Your healthcare provider may recommend that you get a ‘non-live’ (inactivated) vaccine, such as some of the seasonal flu vaccines. If you plan to get a ‘non-live’ (inactivated) vaccine, it should be given, whenever possible, at least 2 weeks before you start Enspryng.
2. Increased liver enzymes.
Your healthcare provider should order blood tests to check your liver enzymes before and while you are taking Enspryng. Your healthcare provider will tell you how often you will need to have these blood tests. Make sure you get all of your follow-up blood tests as ordered by your healthcare provider. Your healthcare provider will tell you if you need to wait to start Enspryng if your liver enzymes are increased.
3.Low neutrophil count.
Enspryng can cause a decrease in your neutrophil counts in your blood. Neutrophils are white blood cells that help the body fight off bacterial infections. Your healthcare provider should order blood tests to check your neutrophil count while you are taking Enspryng.
See “What are the possible side effects with Enspryng?”
Who should not take Enspryng?
Do not take Enspryng if you:
- are allergic to satralizumab-mwge or any of the ingredients in Enspryng. See “What are the ingredients in Enspryng?” at the end of this medication guide for a complete list of ingredients in Enspryng.
- have an active hepatitis B infection.
- have active or untreated inactive (latent) TB.
What should I tell my healthcare provider before taking Enspryng?
Before you take Enspryng, tell your healthcare provider about all of your medical conditions, including if you:
- have or think you have an infection. See “What is the most important information I should know about Enspryng?”
- have liver problems.
- have ever had hepatitis B or are a carrier of the hepatitis B virus.
- have had or have been in contact with someone with tuberculosis.
- have had a recent vaccination or are scheduled to receive any vaccination.
- are pregnant, think that you might be pregnant, or plan to become pregnant. It is not known if Enspryng will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Enspryng passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Enspryng.
Tell your healthcare provider about all the medicines you are taking, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Enspryng?
- Enspryng is provided as a solution in a single-dose, prefilled syringe of 120 mg/mL of satralizumab-mwge.
- See the Instructions for Use inside the carton for complete instructions for the right way to prepare and inject Enspryng.
- Enspryng is given by an injection under the skin (subcutaneously). If your healthcare provider decides that you or your caregiver can give your injections of Enspryng, you or your caregiver should receive training on the right way to prepare and inject Enspryng.
- Always inject all of the medicine in the syringe.
- The first 3 injections (loading period) of Enspryng are taken 1 time every 2 weeks.
- After this, injection of Enspryng is taken every 4 weeks (maintenance period). Keep taking Enspryng 1 time every 4 weeks for as long as your healthcare provider tells you to.
- If you miss a dose of Enspryng, talk to your health care provider about restarting dosing.
What are the possible side effects of Enspryng?
Enspryng may cause serious side effects, including:
- See “What is the most important information I should know about Enspryng?”
- Serious allergic reactions. Serious allergic reactions that may be life-threatening have happened with other medicines like Enspryng. Tell your healthcare provider before taking your next dose if you had hives, rash, or flushing after your injection. Seek medical attention right away if you have any symptoms of a serious allergic reaction, such as:
The most common side effects of Enspryng include:
- sore throat, runny nose (nasopharyngitis)
- rash
- fatigue
- extremity pain
- headache
- upper respiratory tract infection
- nausea
- inflammation of the stomach lining (gastritis)
- joint pain
These are not all the possible side effects of Enspryng.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Genentech at 1-888-835-2555.
General information about the safe and effective use of Enspryng
Medicines are sometimes prescribed for purposes other than those listed in a medication guide. Do not use Enspryng for a condition for which it was not prescribed. Do not give Enspryng to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Enspryng that is written for health professionals.
How should I store Enspryng?
- Store Enspryng in the refrigerator between 36°F to 46°F (2°C to 8°C) in the original carton.
- Protect from light.
- Do not freeze or use the syringe if it has been frozen.
- Do not shake.
- Enspryng, if unopened, can be removed from and returned to the refrigerator, if needed. The total combined time out of the refrigerator should not be more than 8 days at a temperature that does not go above 86°F (30°C).
What are the ingredients in Enspryng?
Active ingredient: satralizumab-mwge
Inactive ingredients: L-arginine, L-histidine, poloxamer 188, L-aspartic acid, and Water for Injection.
For more information, go to www.ENSPRYNG.com or call 1-844-NSPRYNG.
Instructions for use for Enspryng
Talk to your healthcare provider if you have any questions.
Important Information
- Each syringe is prefilled with a medicine called Enspryng.
- Each carton of Enspryng contains only 1 prefilled syringe.
- Each prefilled syringe can be used only 1 time.
Do not share your Enspryng syringe with other people. You may give them a serious infection or get a serious infection from them.
Do not:
- take the needle cap off until you are ready to inject Enspryng.
- use the syringe if it has been dropped or damaged.
- try to take the syringe apart at any time.
- leave the syringe unattended.
- re-use the same syringe.
How should I store the Enspryng prefilled syringe?
Keep the unused syringe in the refrigerator between 36°F to 46°F (2°C to 8°C) until ready to use.
Before giving an injection, if the Enspryng is not opened, it can be removed from and placed back in the refrigerator if needed. The total combined time out of the refrigerator should not be more than 8 days at a temperature that does not go above 86°F (30°C).
Keep the syringe in its original carton away from direct sunlight.
Always keep the syringe dry.
Keep the Enspryng syringe and all medicines out of the reach of children.
Do not:
- freeze the syringe.
- use the syringe if it has been frozen.
- shake.
Supplies needed to give your injection
Each Enspryng carton contains:
- 1 prefilled syringe for 1-time use only.
Not included in the carton:
- 1 alcohol pad
- 1 sterile cotton ball or gauze
- 1 small bandage
- 1 FDA-cleared puncture-resistant sharps container for safe disposal of the needle cap and used syringe. See Step 21 “Disposing of Enspryng” at the end of this Instructions for use.
Enspryng prefilled syringe
(See Figure A and Figure B)
Before use:
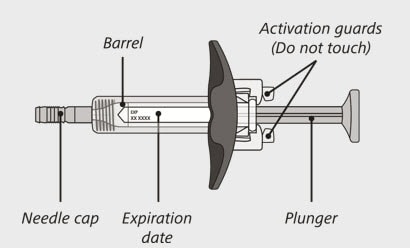
Figure A
After use:
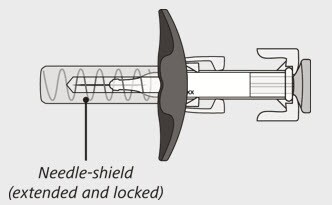
Figure B
The syringe has a needle-shield that automatically covers the needle when the injection is complete.
Prepare to use Enspryng
1. Take the carton containing the syringe out of the refrigerator and place it on a clean, flat work surface (like a table).
2. Check the expiration (EXP) date on the back of the carton (See Figure C). Do not use if the carton has expired.
3. Check the front of the carton to make sure it is sealed (Figure C). Do not use if the seal has been broken.
If the expiration date has passed or the seal is broken, do not use. Then go to Step 21 “Disposing of Enspryng” and contact your healthcare provider.
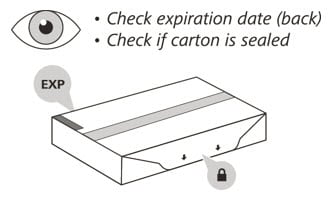
Figure C
4. Open the sealed carton (See Figure D).
Figure D
5. Carefully lift the syringe out of the carton by holding the barrel (See Figure E).
Do not:
- turn the carton upside down to remove the syringe.
- touch the activation guards because this may damage the syringe.
- hold the plunger or needle cap.
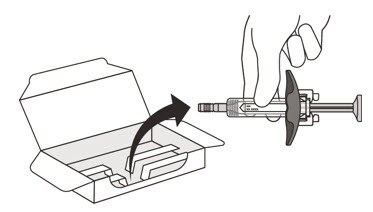
Figure E
Check the syringe
(See Figure F)
6. Check the expiration date on the syringe. Do not use the syringe if it has expired.
7. Check the syringe for any damage. Do not use if it is cracked or broken.
8. Check that the liquid through the viewing window is clear and colorless to slightly yellow. Do not inject the medicine if the liquid is cloudy, discolored, or has particles in it.
- There may be some small air bubbles in the syringe. This is normal and you should not try to remove them.
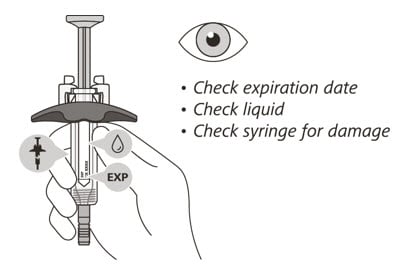
Figure F
If the expiration (EXP) date has passed, the syringe is damaged or the liquid is cloudy, discolored or has particles in it, do not use. Then go to Step 21 “Disposing of Enspryng” and contact your healthcare provider.
Let your syringe warm up
9. After you have checked the syringe, place it on a clean, flat work surface (like a table) for 30 minutes. This will allow it to reach room temperature. (See Figure G).
It is important to let the syringe gently warm up as injecting cold medicine may feel uncomfortable and make it harder to push.
Do not:
- speed up the warming process in any way, such as using a microwave or placing the syringe in warm water.
- remove the needle cover while the syringe is reaching room temperature.
Figure G
Wash your hands
10. Wash your hands with soap and water. (See Figure H).
Figure H
Choose the injection site
11. Choose your injection site in either:
- the lower part of your stomach (abdomen) or
- the front and middle of your thighs. (See Figure I).
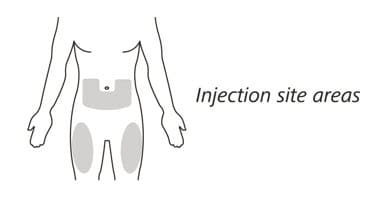
Figure I
- Do not inject into the 2-inch (5 cm) area around your belly button.
- Do not inject into moles, scars, bruises, or areas where the skin is tender, red, hard or broken.
Choose a different injection site for each new injection. Choose a different place to inject which is at least 1 inch (2.5 cm) away from the place where you last injected.
Clean the injection site
12. Wipe the injection site with an alcohol pad and let it air dry.
Do not:
- fan or blow on the area which you have cleaned.
- touch the injection site again before you give the injection.
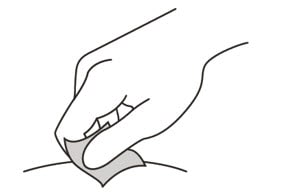
Figure J
Inject Enspryng
13. Hold the barrel of the syringe between your thumb and index finger. With your other hand, pull the needle cap straight off. You may see a drop of liquid at the end of the needle. This is normal and will not affect your dose (See Figure K).
- Use the syringe within 5 minutes of removing the cap or the needle may clog.
Do not:- take the needle cap off until you are ready to inject Enspryng.
- put the needle cap back on after it has been removed as this may damage the needle.
- touch the needle or let it touch any surfaces after removing the needle cap.
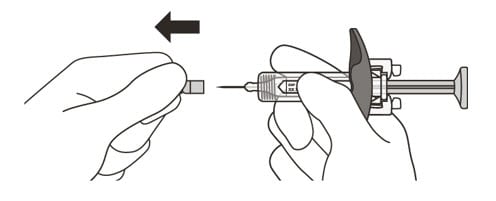
Figure K
14. Throw away the needle cap in a puncture-resistant sharps container immediately. See Step 21 “Disposing of Enspryng”.
15. Hold the barrel of the syringe using your thumb and index finger. With your other hand, pinch the area of skin you have cleaned (See Figure L).
16. Use a quick, dart-like motion to insert the needle at an angle between 45° to 90° (See Figure L).
Do not:
- insert the needle through clothing.
- change the angle of the injection.
- insert the needle again.
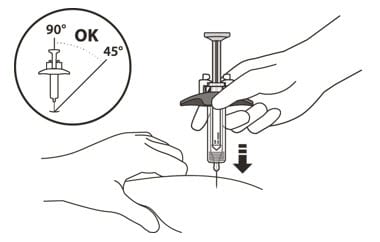
Figure L
17. After the needle is inserted, let go of the pinched skin.
18. Slowly inject all of the medicine by gently pushing the plunger all the way down until it touches the activation guards (See Figure M).
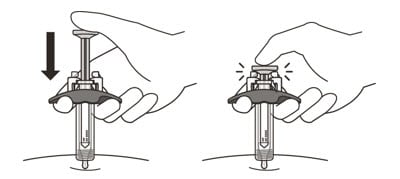
Figure M
19. Gently release the plunger and allow the needle to come out of the skin at the same angle it was inserted (See Figure N).
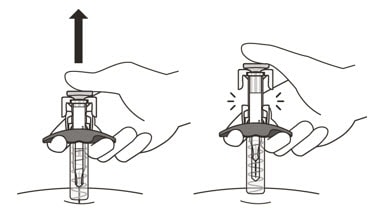
Figure N
- The needle will now be covered by the needle-shield. If the needle is not covered, carefully place the syringe into a puncture-resistant sharps container to avoid injury. See Step 21 “Disposing of Enspryng”.
Taking care of the injection site
20. There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site but do not rub it. If needed, you may also cover the area you injected with a small bandage. If the medicine gets into contact with your skin, wash the area with water.
Disposing of Enspryng
21. Put your used syringe in an FDA-cleared sharps disposal container immediately after use (See Figure O). Do not throw away (dispose of) the syringe in your household trash.
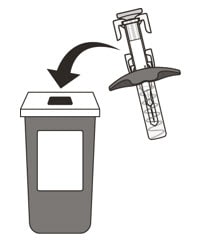
Figure O
- If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out
- upright and stable during use
- leak-resistant
- properly labeled to warn of hazardous waste inside the container
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about the safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in trash unless your community guidelines permit this.
- Do not recycle your used sharps disposal container.
Label
PRINCIPAL DISPLAY PANEL – 120 MG/ML SYRINGE CARTON
- NDC 50242-007-01
- Rx only
- Enspryng®
(satralizumab-mwge)
Injection - 120 mg/mL
- For Subcutaneous Use.
Single-Dose Prefilled Syringe. - Refrigerate at 2°C to 8°C (36°F to 46°F) in original carton to protect from light.
Do not freeze. Do not shake. - ATTENTION PHARMACIST: Each patient is required to receive the enclosed
Medication Guide. - 1 prefilled syringe
- Genentech
- 10240933

SRC: NLM .
