Enhertu
Generic name: fam-trastuzumab deruxtecan
Dosage form: intravenous infusion
Drug class: HER2 inhibitors
Medically reviewed by A Ras MD.
What is Enhertu?
Enhertu is a prescription medicine used in adults to treat human epidermal growth factor receptor 2 (HER2)-positive breast cancer that cannot be removed by surgery or that has spread to other parts of your body (metastatic), and who have received two or more prior anti-HER2 breast cancer treatments, stomach cancer called gastric or gastroesophageal junction (GEJ) adenocarcinoma that has spread to areas near your stomach (locally advanced) or that has spread to other parts of your body (metastatic), and who have received a prior trastuzumab-based regimen.
It is not known if Enhertu is safe and effective in children.
Description
Fam-trastuzumab deruxtecan-nxki is a HER2-directed antibody and topoisomerase inhibitor conjugate. Fam-trastuzumab deruxtecan-nxki is an antibody-drug conjugate (ADC) composed of three components: 1) a humanized anti-HER2 IgG1 monoclonal antibody (mAb), covalently linked to 2) a topoisomerase inhibitor, via 3) a tetrapeptide-based cleavable linker. Deruxtecan is composed of a protease-cleavable maleimide tetrapeptide linker and the topoisomerase inhibitor, DXd, which is an exatecan derivative.
The antibody is produced in Chinese hamster ovary cells by recombinant DNA technology, and the topoisomerase inhibitor and linker are produced by chemical synthesis. Approximately 8 molecules of deruxtecan are attached to each antibody molecule. Fam-trastuzumab deruxtecan-nxki has the following structure:
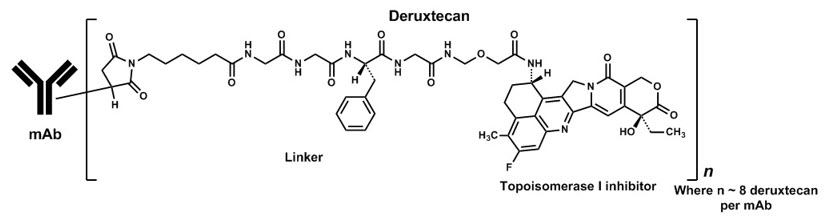
ENHERTU (fam-trastuzumab deruxtecan-nxki) is a sterile, white to yellowish white, preservative-free lyophilized powder in single-dose vials. Each vial delivers 100 mg of fam-trastuzumab deruxtecan-nxki, L-histidine (4.45 mg), L-histidine hydrochloride monohydrate (20.2 mg), polysorbate 80 (1.5 mg), and sucrose (450 mg). Following reconstitution with 5 mL of Sterile Water for Injection, USP, the resulting concentration of fam-trastuzumab deruxtecan-nxki is 20 mg/mL with a pH of 5.5. The resulting solution is administered by intravenous infusion following dilution.
\
Mechanism of Action
Fam-trastuzumab deruxtecan-nxki is a HER2-directed antibody-drug conjugate. The antibody is a humanized anti-HER2 IgG1. The small molecule, DXd, is a topoisomerase I inhibitor attached to the antibody by a cleavable linker. Following binding to HER2 on tumor cells, fam-trastuzumab deruxtecan-nxki undergoes internalization and intracellular linker cleavage by lysosomal enzymes. Upon release, the membrane-permeable DXd causes DNA damage and apoptotic cell death.
What is the most important information I should know about Enhertu?
Enhertu can cause serious side effects, including:
- Lung problems that may be severe, life-threatening or that may lead to death. If you develop lung problems your healthcare provider may treat you with corticosteroid medicines. Tell your healthcare provider right away if you get any of the following signs and symptoms:
- cough
- trouble breathing or shortness of breath
- fever
- other new or worsening breathing symptoms (e.g., chest tightness, wheezing)
- Low white blood cell count (neutropenia). Low white blood cell counts are common with Enhertu and can sometimes be severe. Your healthcare provider will check your white blood cell counts before starting Enhertu and before starting each dose. Tell your healthcare provider right away if you develop any signs or symptoms of an infection or have fever or chills during treatment with Enhertu.
- Heart problems that may affect your heart’s ability to pump blood. Your healthcare provider will check your heart function before starting treatment with Enhertu. Tell your healthcare provider right away if you get any of the following signs and symptoms:
- new or worsening shortness of breath
- coughing
- feeling tired
- swelling of your ankles or legs
- irregular heartbeat
- sudden weight gain
- dizziness or feeling light-headed
- loss of consciousness
Your healthcare provider will check you for these side effects during your treatment with Enhertu. Your healthcare provider may reduce your dose, delay treatment or completely stop treatment with Enhertu if you have severe side effects.
- Harm to your unborn baby. Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with Enhertu.
- If you are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with Enhertu.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with Enhertu and for at least 7 months after the last dose.
- Males who have female partners that are able to become pregnant should use effective birth control (contraception) during treatment with Enhertu and for at least 4 months after the last dose.
See “What are the possible side effects of Enhertu?” for more information about side effects.
What should I tell my healthcare provider before using Enhertu?
Before you receive Enhertu, tell your healthcare provider about all of your medical conditions, including if you:
- have lung or breathing problems.
- have signs or symptoms of an infection.
- have or have had any heart problems.
- are breastfeeding or plan to breastfeed. It is not known if Enhertu passes into your breast milk. Do not breastfeed during treatment with Enhertu and for 7 months after the last dose.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Enhertu?
- You will receive Enhertu into your vein through an intravenous (IV) line by your healthcare provider.
- Enhertu is given 1 time every three weeks (21-day treatment cycle).
- Your healthcare provider will decide how many treatments you need.
- Your healthcare provider may slow down or temporarily stop your infusion of Enhertu if you have an infusion-related reaction, or permanently stop Enhertu if you have severe infusion reactions.
- If you miss a planned dose of Enhertu, call your healthcare provider right away to schedule an appointment. Do not wait until the next planned treatment cycle.
What are the possible side effects of Enhertu?
Enhertu can cause serious side effects. See “What is the most important information I should know about Enhertu?”
The most common side effects of Enhertu, when used in people with breast cancer, include:
- nausea
- low white blood cell counts
- low red blood cell counts
- feeling tired
- vomiting
- hair loss
- increased liver function tests
- low platelet counts
- constipation
- decreased appetite
- diarrhea
- low levels of blood potassium
- cough
The most common side effects of Enhertu, when used in people with stomach cancer, include:
- low red blood cell counts
- low white blood cell counts
- low platelet counts
- nausea
- decreased appetite
- increased liver function tests
- feeling tired
- diarrhea
- low levels of blood potassium
- vomiting
- constipation
- fever
- hair loss
Enhertu may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of Enhertu. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Enhertu
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about Enhertu that is written for professionals.
What are the ingredients in Enhertu?
Active Ingredient: fam-trastuzumab deruxtecan-nxki.
Inactive Ingredient: L-histidine, L-histidine hydrochloride monohydrate, polysorbate 80, and sucrose.
Label
PRINCIPAL DISPLAY PANEL – 100 MG VIAL CARTON
- NDC 65597-406-01
Rx only - ENHERTU®
(fam-trastuzumab deruxtecan-nxki) - For Injection
- 100 mg per vial
- For Intravenous Infusion Only
Dispense the enclosed Medication Guide to each patient.
Reconstitute and Dilute prior to administration
Single-Dose Vial
Discard Unused Portion - Hazardous Drug
- KEEP REFRIGERATED
- 1 vial
- Daiichi-Sankyo
AstraZeneca 
SRC: NLM .
