Emend Injection
Generic name: aprepitant (oral/injection)
Drug class: NK1 receptor antagonists
Medically reviewed by A Ras MD.
What is Emend injection?
Emend injection is a prescription medicine used with other medicines that treat nausea and vomiting in patients 6 months of age and older to prevent nausea and vomiting caused by certain anti-cancer (chemotherapy) medicines.
Emend for injection is not used to treat nausea and vomiting that you already have. It is not known if Emend for injection is safe and effective in children less than 6 months of age.
Description
EMEND (fosaprepitant) for injection is a sterile, lyophilized formulation containing fosaprepitant dimeglumine, a prodrug of aprepitant, a substance P/neurokinin-1 (NK1) receptor antagonist, an antiemetic agent, chemically described as 1-Deoxy-1-(methylamino)-D-glucitol[3-[[(2R,3S)-2-[(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-3-(4-fluorophenyl)-4-morpholinyl]methyl]-2,5-dihydro-5-oxo-1H-1,2,4-triazol-1-yl]phosphonate (2:1) (salt).
Its empirical formula is C23H22F7N4O6P ∙ 2(C7H17NO5) and its structural formula is:
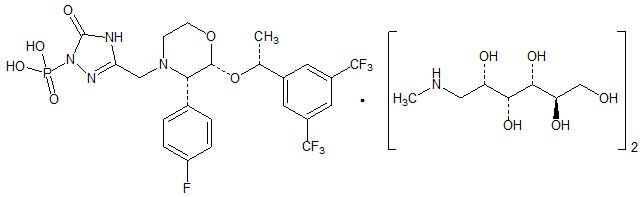
Fosaprepitant dimeglumine is a white to off-white amorphous powder with a molecular weight of 1004.83. It is freely soluble in water.
Each vial of EMEND for injection for administration as an intravenous infusion contains 150 mg of fosaprepitant (equivalent to 245.3 mg of fosaprepitant dimeglumine) and the following inactive ingredients: edetate disodium (18.8 mg), polysorbate 80 (75 mg), lactose anhydrous (375 mg), sodium hydroxide and/or hydrochloric acid (for pH adjustment).
Mechanism of Action
Fosaprepitant is a prodrug of aprepitant and accordingly, its antiemetic effects are attributable to aprepitant.
Aprepitant is a selective high-affinity antagonist of human substance P/neurokinin 1 (NK1) receptors. Aprepitant has little or no affinity for serotonin (5-HT3), dopamine, and corticosteroid receptors, the targets of existing therapies for chemotherapy-induced nausea and vomiting (CINV). Aprepitant has been shown in animal models to inhibit emesis induced by cytotoxic chemotherapeutic agents, such as cisplatin, via central actions.
Animal and human Positron Emission Tomography (PET) studies with aprepitant have shown that it crosses the blood brain barrier and occupies brain NK1 receptors. Animal and human studies show that aprepitant augments the antiemetic activity of the 5-HT3-receptor antagonist ondansetron and the corticosteroid dexamethasone and inhibits both the acute and delayed phases of cisplatin-induced emesis.
Who should not use Emend for injection?
Do not receive Emend for injection if you:
- are allergic to fosaprepitant, aprepitant, or any of the ingredients in Emend for injection. See the end of this guide for a complete list of the ingredients in Emend for injection.
- are taking pimozide (Orap)
What should I tell my healthcare provider before using Emend for injection?
Before receiving Emend for injection, tell your healthcare provider if you:
- have liver problems
- are pregnant or plan to become pregnant. It is not known if Emend for injection can harm your unborn baby.
- Women who use birth control medicines containing hormones to prevent pregnancy (birth control pills, skin patches, implants, and certain IUDs) should also use a backup method of birth control that does not contain hormones, such as condoms and spermicides, during treatment with Emend for injection and for 1 month after receiving Emend for injection.
- are breastfeeding or plan to breastfeed. It is not known if Emend for injection passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive Emend for injection.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Emend for injection may affect the way other medicines work, and other medicines may affect the way Emend for injection works, causing serious side effects.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I use Emend for injection?
Adults 18 years of age and older:
Emend for injection will be given on Day 1 of chemotherapy treatment. It will be given to you by intravenous (IV) infusion in your vein about 50 to 60 minutes before you start your chemotherapy treatment.
Children 6 months to 17 years of age:
Emend for injection will be given on Day 1 of chemotherapy treatment. It will be given to your child by intravenous (IV) infusion into a large vein through a type of IV line called a central venous catheter, about 1 hour to 1 ½ hours before the start of their chemotherapy treatment.
- Your child may also receive:
- capsules of Emend or an oral suspension of Emend on Days 2 and 3. If your child will receive either of these, see the Patient Information for Emend capsules or Emend for oral suspension for further information.
If you take the blood thinner medicine warfarin sodium (Coumadin, Jantoven), your healthcare provider may do blood tests after you receive Emend for injection to check your blood clotting.
What are the possible side effects of Emend for injection?
Emend for injection may cause serious side effects, including:
- Serious allergic reactions. Allergic reactions can happen with Emend for injection and may be serious. Tell your doctor or nurse right away if you have hives, rash, itching, flushing or redness of your face or skin, trouble breathing or swallowing, dizziness, a rapid or weak heartbeat, or you feel faint during or soon after you receive Emend for injection, as you may need emergency medical care.
- Severe skin reactions, which may include rash, skin peeling, or sores, may occur.
- Infusion site reactions (ISR) at or near the infusion site have happened with Emend for Injection.
Most severe ISR have happened with a certain type of chemotherapy medicine that can burn or blister your skin (vesicant) with side effects, including pain, swelling and redness. Death of skin tissue (necrosis) has happened in some people getting this type of chemotherapy medicine. Most ISR can happen with the first, second, or third dose and some can last up to 2 weeks or longer. Tell your healthcare provider right away if you get any infusion site side effects.
In adults, the most common side effects of Emend for injection include:
- tiredness
- diarrhea
- low white blood cell and red blood cell counts
- weakness
- feeling weak or numb in your arms and legs
- painful, difficult, or changes in your digestion (dyspepsia)
- urinary tract infection
- pain in your arms and legs
In children 6 months to 17 years of age, the most common side effects of Emend for injection include:
- low red blood cell count
- low white blood cell count
- low blood platelet count
- low white blood cell count with a fever
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of Emend for injection. For more information ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Emend for injection
If you would like more information about Emend for injection, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Emend for injection that is written for health professionals. For more information about Emend for injection call 1-800-622-4477 or go to www.emend.com.
How should I store Emend for injection?
Emend for injection vials must be refrigerated, store at 2°C-8°C (36°F-46°F).
The reconstituted final drug solution is stable for 24 hours at ambient room temperature [at or below 25°C (77°F)]. Discard unused portion.
What are the ingredients in Emend for injection?
Active ingredient: fosaprepitant
Inactive ingredients: edetate disodium, polysorbate 80, lactose anhydrous, sodium hydroxide and/or hydrochloric acid (for pH adjustment)
Label
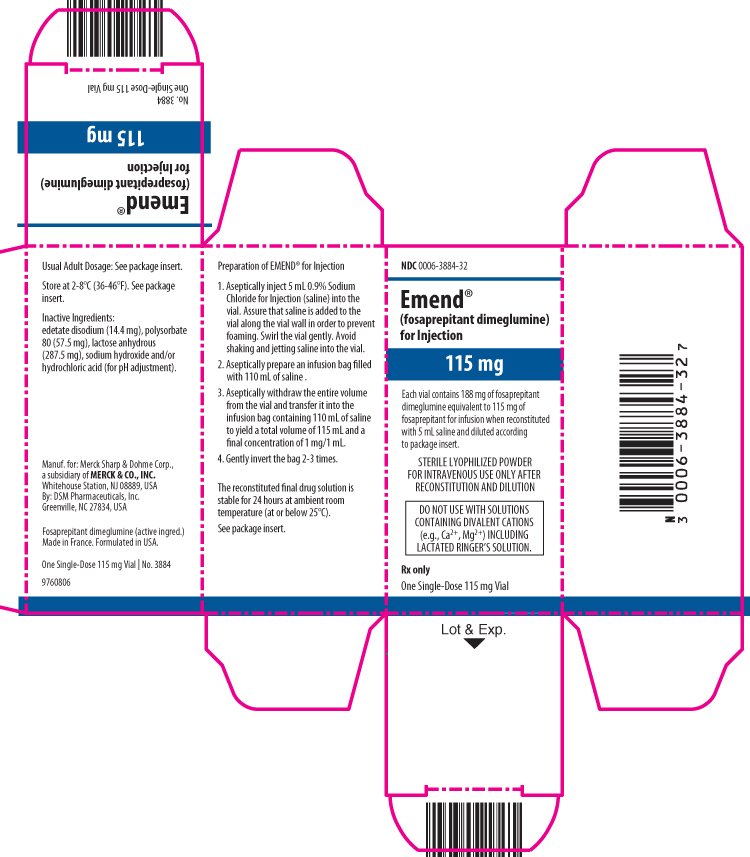
PRINCIPAL DISPLAY PANEL – CARTON 115 MG
- NDC 0006-3884-32
- Emend®
(fosaprepitant dimeglumine)
for Injection - 115 mg
- Each vial contains 188 mg of fosaprepitant dimeglumine equivalent to 115 mg of fosaprepitant for infusion when reconstituted with 5 mL saline and diluted according to package insert.
- STERILE LYOPHILIZED POWDER FOR INTRAVENOUS USE ONLY AFTER RECONSTITUTION AND DILUTION
- DO NOT USE WITH SOLUTIONS CONTAINING DIVALENT CATIONS (e.g., Ca2+, Mg2+) INCLUDING LACTATED RINGER’S SOLUTION.
- Rx only
- One Single-Dose 115 mg Vial
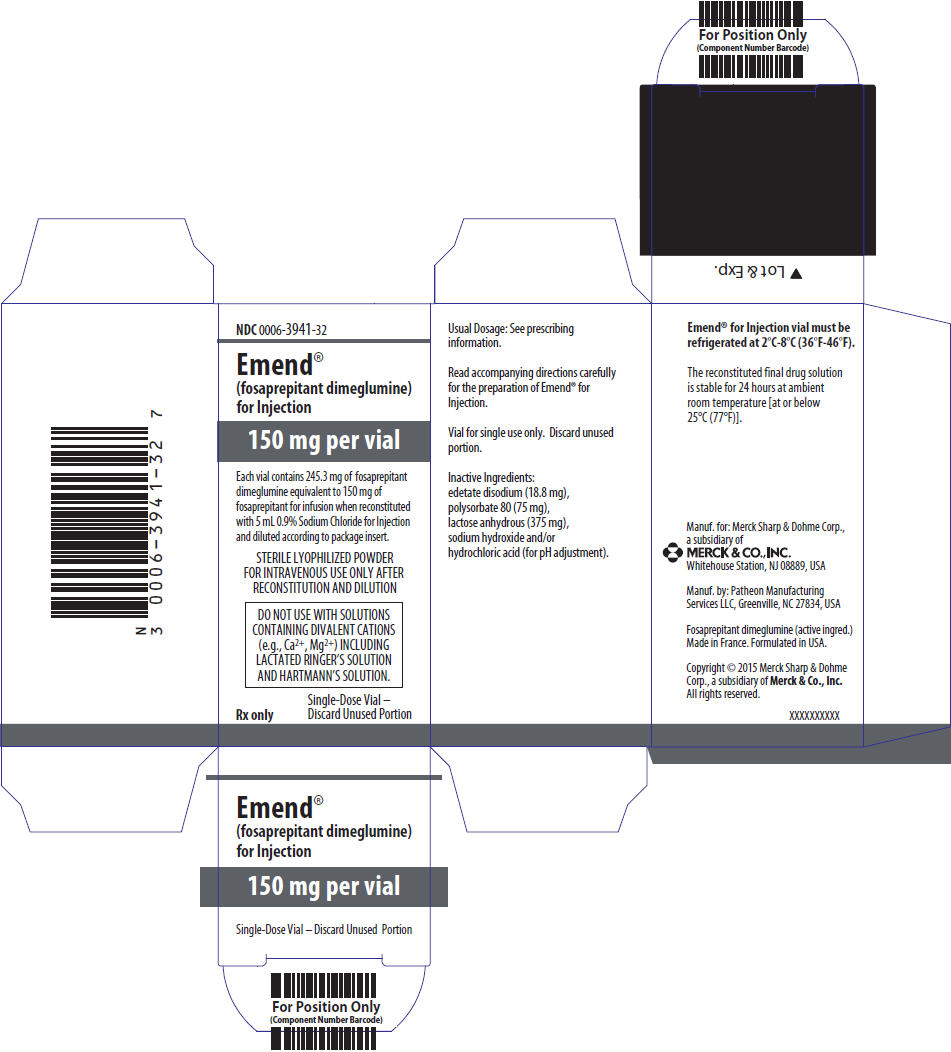
PRINCIPAL DISPLAY PANEL – 150 MG VIAL CARTON
- NDC 0006-3941-32
- Emend®
(fosaprepitant dimeglumine)
for Injection - 150 mg per vial
- Each vial contains 245.3 mg of fosaprepitant
dimeglumine equivalent to 150 mg of
fosaprepitant for infusion when reconstituted
with 5 mL 0.9% Sodium Chloride for Injection
and diluted according to package insert. - STERILE LYOPHILIZED POWDER
FOR INTRAVENOUS USE ONLY AFTER
RECONSTITUTION AND DILUTION - DO NOT USE WITH SOLUTIONS
CONTAINING DIVALENT CATIONS
(e.g., Ca2+, Mg2+) INCLUDING
LACTATED RINGER’S SOLUTION
AND HARTMANN’S SOLUTION. - Rx only
- Single-Dose Vial –
Discard Unused Portion
SRC: NLM .
