Elvitegravir
Generic name: Elvitegravir
Brand name: Vitekta
Drug class: Integrase strand transfer inhibitor
Medically reviewed by A Ras MD.
What is elvitegravir?
Elvitegravir is a prescription medication medicine that is used to treat HIV infection.
Description
GENVOYA (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) is a fixed-dose combination tablet containing elvitegravir (EVG), cobicistat (COBI), emtricitabine (FTC), and tenofovir alafenamide (TAF) for oral administration.
- EVG is an HIV-1 integrase strand transfer inhibitor.
- COBI is a mechanism-based inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family.
- FTC, a synthetic nucleoside analog of cytidine, is an HIV nucleoside analog reverse transcriptase inhibitor (HIV NRTI).
- TAF, an HIV NRTI, is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate.
Each tablet contains 150 mg of EVG, 150 mg of COBI, 200 mg of FTC, and 10 mg of TAF (equivalent to 11.2 mg of tenofovir alafenamide fumarate). The tablets include the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, silicon dioxide, and sodium lauryl sulfate. The tablets are film-coated with a coating material containing FD&C Blue No. 2/indigo carmine aluminum lake, iron oxide yellow, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Elvitegravir: The chemical name of elvitegravir is 6-(3-chloro-2-fluorobenzyl)-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
It has a molecular formula of C23H23ClFNO5 and a molecular weight of 447.88. It has the following structural formula:
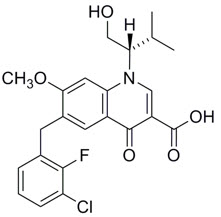
Elvitegravir is a white to pale yellow powder with a solubility of less than 0.3 micrograms per mL in water at 20 °C.
Cobicistat: The chemical name for cobicistat is 2,7,10,12-tetraazatridecanoic acid, 12-methyl-13-[2-(1-methylethyl)-4-thiazolyl]-9-[2-(4-morpholinyl)ethyl]-8,11-dioxo-3,6-bis(phenylmethyl)-, 5-thiazolylmethyl ester, (3R,6R,9S)-.
It has a molecular formula of C40H53N7O5S2 and a molecular weight of 776.02. It has the following structural formula:
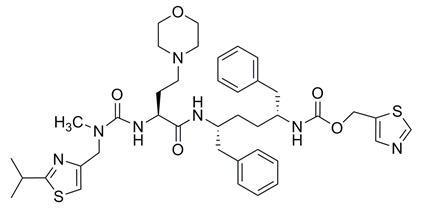
Cobicistat is adsorbed onto silicon dioxide. Cobicistat on silicon dioxide drug substance is a white to pale yellow powder with a solubility of 0.1 mg per mL in water at 20 °C.
Emtricitabine: The chemical name of emtricitabine is 4-amino-5-fluoro-1-(2R-hydroxymethyl-1,3-oxathiolan-5S-yl)-(1H)-pyrimidin-2-one. Emtricitabine is the (-)-enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5 position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
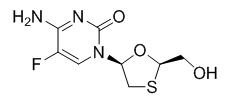
Emtricitabine is a white to off-white powder with a solubility of approximately 112 mg per mL in water at 25 °C.
Tenofovir alafenamide (TAF): The chemical name of tenofovir alafenamide fumarate drug substance is L-alanine, N-[(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-, 1-methylethyl ester, (2E)-2-butenedioate (2:1).
It has an empirical formula of C21H29O5N6P∙½(C4H4O4) and a formula weight of 534.5. It has the following structural formula:
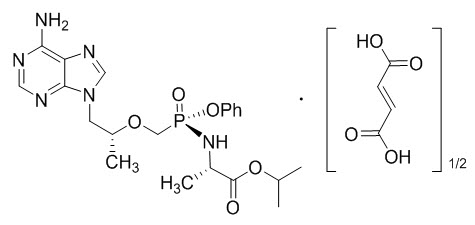
Tenofovir alafenamide fumarate is a white to off-white or tan powder with a solubility of 4.7 mg per mL in water at 20 °C.
Mechanism of Action
GENVOYA is a fixed-dose combination of antiretroviral drugs elvitegravir (plus the CYP3A inhibitor cobicistat), emtricitabine, and tenofovir alafenamide
Before taking elvitegravir, tell your doctor:
- If you are allergic to elvitegravir; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have liver disease.
- If you are taking cobicistat and you are also taking certain other drugs to treat HIV. There are many drugs that are not to be taken if you are taking elvitegravir along with cobicistat. Ask your doctor or pharmacist if you are not sure.
- If you are taking any of these drugs: Boceprevir, carbamazepine, efavirenz, nevirapine, oxcarbazepine, phenobarbital, phenytoin, rifampin, rifapentine, or St. John’s wort.
- If you are taking another drug that has the same drug in it.
- If you are breast-feeding. Do not breast-feed while you take elvitegravir.
This is not a list of all drugs or health problems that interact with elvitegravir.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take elvitegravir with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take elvitegravir?
- Tell all of your health care providers that you take elvitegravir. This includes your doctors, nurses, pharmacists, and dentists.
- This medicine interacts with many other drugs. The chance of elvitegravir’s side effects may be raised or how well elvitegravir works may be lowered. The chance of the other drugs’ side effects may also be raised. This may include very bad, life-threatening, or deadly side effects. Check with your doctor and pharmacist to make sure that it is safe for you to take elvitegravir with all of your other drugs (prescription or OTC, natural products, vitamins).
- This medicine is not a cure for HIV. Stay under the care of your doctor.
- This medicine does not stop the spread of diseases like HIV or hepatitis that are passed through blood or having sex. Do not have any kind of sex without using a latex or polyurethane condom. Do not share needles or other things like toothbrushes or razors.
- Birth control pills and other hormone-based birth control may not work as well to prevent pregnancy. Use some other kind of birth control also like a condom when taking elvitegravir.
- Tell your doctor if you are pregnant or plan on getting pregnant. You will need to talk about the benefits and risks of using elvitegravir while you are pregnant.
How is elvitegravir best taken?
Use elvitegravir as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Take elvitegravir with food.
- If you are also taking didanosine, take it at least 1 hour before or 2 hours after elvitegravir.
- Do not take antacids within 2 hours before or 2 hours after taking elvitegravir.
- Keep taking elvitegravir as you have been told by your doctor or other health care provider, even if you feel well.
- It is important that you do not miss or skip a dose of elvitegravir during treatment.
What do I do if I miss a dose?
- Take a missed dose as soon as you think about it.
- If it is close to the time for your next dose, skip the missed dose and go back to your normal time.
- Do not take 2 doses at the same time or extra doses.
- If you are not sure what to do if you miss a dose, call your doctor.
What are the side effects of elvitegravir that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Changes in your immune system can happen when you start taking drugs to treat HIV. If you have an infection that you did not know you had, it may show up when you take elvitegravir. Tell your doctor right away if you have any new signs after you start elvitegravir, even after taking it for several months. This includes signs of infection like fever, sore throat, weakness, cough, or shortness of breath.
What are some other side effects of elvitegravir?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out elvitegravir?
- Store in the original container at room temperature.
- Store in a dry place. Do not store in a bathroom.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
PRINCIPAL DISPLAY PANEL – 30 TABLET BOTTLE LABEL
- NDC 61958-1901-1
30 tablets - Genvoya®
(elvitegravir, cobicistat,
emtricitabine, and tenofovir
alafenamide) Tablets
150 mg/150 mg/200 mg/10 mg - Note to pharmacist:
Do not cover ALERT box with pharmacy label. - ALERT: Find out about medicines that
should NOT be taken with Genvoya®

SRC: NLM .
