Duexis
Generic name: famotidine and ibuprofen
Drug class: Nonsteroidal anti-inflammatory drugs
Medically reviewed by A Ras MD.
What is Duexis?
Duexis is a prescription medicine used torelieve the signs and symptoms of rheumatoid arthritis and osteoarthritis, decrease the risk of developing ulcers of the stomach and upper intestines (upper gastrointestinal ulcers) in people taking ibuprofen for rheumatoid arthritis and osteoarthritis.
It is not known if Duexis is safe and effective in children.
Description
DUEXIS (ibuprofen and famotidine) is supplied as a tablet for oral administration which combines the nonsteroidal anti- inflammatory drug, ibuprofen, and the histamine H2-receptor antagonist, famotidine.
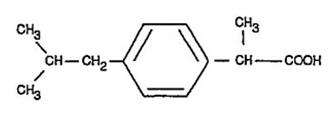
Ibuprofen is (±)-2-(p-isobutylphenyl)propionic acid. Its chemical formula is C13H18O2 and molecular weight is 206.28. Ibuprofen is a white powder that is very slightly soluble in water (<1 mg/mL) and readily soluble in organic solvents such as ethanol and acetone. Its structural formula is:
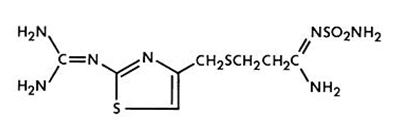
Famotidine is N’-(aminosulfonyl)-3-[[[2-[(diaminomethylene)amino]-4-thiazolyl]methyl]thio]propanimidamide. Its chemical formula is C8H15N7O2S3 and molecular weight is 337.45. Famotidine is a white to pale yellow crystalline compound that is freely soluble in glacial acetic acid, slightly soluble in methanol, very slightly soluble in water, and practically insoluble in ethanol. Its structural formula is:
Each DUEXIS tablet contains ibuprofen, USP (800 mg) and famotidine, USP (26.6 mg). The inactive ingredients in DUEXIS include: microcrystalline cellulose, anhydrous lactose, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, purified water, povidone, titanium dioxide, polyethylene glycol, polysorbate 80, polyvinyl alcohol, hypromellose, talc, FD&C Blue #2/Indigo Carmine Aluminum Lake, and FD&C Blue #1/Brilliant Blue FCF Aluminum Lake.
Mechanism of Action
DUEXIS is a fixed-combination tablet of ibuprofen and famotidine. The ibuprofen component has analgesic, anti- inflammatory, and antipyretic properties. The mechanism of action of the ibuprofen component of DUEXIS, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Ibuprofen is a potent inhibitor of prostaglandin synthesis in vitro. Ibuprofen concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because ibuprofen is an inhibitor of prostaglandin synthesis, its mode of action may be due to an increase of prostaglandins in peripheral tissues.
Famotidine is a competitive inhibitor of histamine H2-receptors. The primary clinically important pharmacologic activity of famotidine is inhibition of gastric secretion. Both the acid concentration and volume of gastric secretion are suppressed by famotidine, while changes in pepsin secretion are proportional to volume output.
Systemic effects of famotidine in the CNS, cardiovascular, respiratory, or endocrine systems were not noted in clinical pharmacology studies. Also, no antiandrogenic effects were noted. Serum hormone levels, including prolactin, cortisol, thyroxine (T4), and testosterone, were not altered after treatment with famotidine.
What is the most important information I should know about Duexis?
Duexis can cause serious side effects including:
- Increased risk of a heart attack or stroke that can lead to death. This risk may happen early in treatment and may increase:
- with increasing doses of medicine containing NSAIDs
- with longer use of medicine containing NSAIDs
Do not take Duexis right before or after a heart surgery called a “coronary artery bypass graft (CABG).” Avoid taking Duexis after a recent heart attack, unless your healthcare provider tells you to. You may have an increased risk of another heart attack if you take Duexis after a recent heart attack.
- Increased risk of bleeding, ulcers, and tears (perforation) of the esophagus (tube leading from the mouth to the stomach), stomach and intestines:
- anytime during use
- without warning symptoms
- that may cause death
The risk of getting an ulcer or bleeding increases with:
- past history of stomach ulcers, or stomach or intestinal bleeding with the use of NSAIDs
- taking medicines called “corticosteroids”, “anticoagulants”, “SSRIs”, or “SNRIs”
- increasing doses of NSAIDs
- longer use of NSAIDs
- smoking
- drinking alcohol
- older age
- poor health
- advanced liver disease
- bleeding problems
You should take Duexis exactly as prescribed, at the lowest dose possible and for the shortest time needed. Duexis contains a non-steroidal anti-inflammatory drug NSAID (ibuprofen). Do not use Duexis with other medicines to lessen pain or fever or with other medicines for colds or sleeping problems without talking to your healthcare provider first, because they may contain an NSAID also.
Duexis may help your acid-related symptoms, but you could still have serious stomach problems. Talk with your healthcare provider.
Duexis contains ibuprofen, an NSAID and famotidine, a histamine H2-receptor blocker medicine.
Who should not take Duexis?
- if you are allergic to ibuprofen, famotidine, any other histamine H2-receptor blocker, or any of the ingredients in Duexis. See the end of this medication guide for a complete list of ingredients.
- if you have had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAIDs.
- right before or after heart bypass surgery.
What should I tell my healthcare provider before taking Duexis?
Before taking Duexis, tell your healthcare provider about all of your medical conditions, including if you:
- have liver or kidney problems.
- have high blood pressure.
- have heart problems.
- have asthma.
- have bleeding problems.
- are pregnant or plan to become pregnant. Talk to your healthcare provider if you are considering taking Duexis during pregnancy. You should not take Duexis after 29 weeks of pregnancy.
- are breastfeeding or plan to breast feed. Ibuprofen and famotidine can pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Duexis.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Duexis and some other medicines can interact with each other and cause serious side effects. Do not start taking any new medicine without talking to your healthcare provider first.
How should I take Duexis?
- Take Duexis exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how many Duexis to take and when to take it.
- Do not change your dose or stop Duexis without first talking to your healthcare provider.
- Swallow Duexis tablets whole with liquid. Do not split, chew, crush or dissolve the Duexis tablet. Tell your healthcare provider if you cannot swallow the tablet whole. You may need a different medicine.
- If you forget to take your dose of Duexis, take it as soon as you remember. If it is almost time for your next dose, do not take the missed dose. Take the next dose on time. Do not take 2 doses at one time to make up for a missed dose.
- You should not take an ibuprofen tablet and famotidine tablet together instead of taking Duexis, because they will not work in the same way.
What are the possible side effects of Duexis?
Duexis can cause serious side effects, including:
- See “What is the most important information I should know about Duexis?”
- heart attack
- stroke
- liver problems including liver failure
- new or worse high blood pressure
- heart failure
- kidney problems including kidney failure
- life-threatening allergic reactions
- asthma attacks in people who have asthma
- life-threatening skin reactions
- low red blood cells (anemia)
Other side effects of Duexis include:
- stomach pain
- constipation
- diarrhea
- gas
- heartburn
- nausea,
- vomiting
- dizziness
Get emergency help right away if you get any of the following symptoms:
- shortness of breath
- chest pain
- weakness in one part or side of your body
- slurred speech
- swelling of the face or throat
Stop taking Duexis and call your healthcare provider right away if you get any of the following symptoms:
- nausea
- more tired or weaker than usual
- diarrhea
- itching
- your skin or eyes look yellow
- indigestion or stomach pain
- flu-like symptoms
- vomit blood
- there is blood in your bowel movement or it is black and sticky like tar
- unusual weight gain
- skin rash or blisters with fever
- swelling of the arms, legs, hands, and feet
If you take too much Duexis, call your poison control center at 1-800-222-1222.
These are not all the possible side effects of Duexis. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about NSAIDs
- Aspirin is an NSAID but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some NSAIDs are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days
General information about the safe and effective use of Duexis
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Duexis for a condition for which it was not prescribed. Do not give Duexis to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about NSAIDs that is written for health professionals.
How do I store Duexis?
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F).
What are the ingredients in Duexis?
Active ingredients: ibuprofen and famotidine
Inactive ingredients: microcrystalline cellulose, anhydrous lactose, croscarmellose sodium, colloidal silicon dioxide, magnesium stearate, purified water, povidone, titanium dioxide, polyethylene glycol, polysorbate 80, polyvinyl alcohol, hypromellose, talc, FD&C Blue#2/Indigo Carmine Aluminum Lake, and FD&C Blue #1/Brilliant Blue FCF Aluminum Lake.
Label
PRINCIPAL DISPLAY PANEL – 800 MG/26.6 MG TABLET BOTTLE LABEL
- NDC 75987-010-03
- DUEXIS®
(ibuprofen and famotidine) Tablets - 800 mg/26.6 mg
Rx Only - 90 TABLETS
- ATTENTION PHARMACIST: Dispense
attached Medication Guide to each patient. - HORIZON


SRC: NLM .
