Duavee
Generic name: bazedoxifene and conjugated estrogens
Drug class: Sex hormone combinations
Medically reviewed by A Ras MD.
What is Duavee?
Duavee is a prescription medicine that contains a mixture of estrogens and bazedoxifene.
Duavee is used after menopause for women with a uterus to reduce moderate to severe hot flushes Estrogens are hormones made by a woman’s ovaries. The ovaries normally stop making estrogens when a woman is between 45 and 55 years old. This drop in body estrogen levels causes the “change of life” or menopause (the end of monthly menstrual periods). Sometimes both ovaries are removed during an operation before natural menopause takes place. The sudden drop in estrogen levels causes “surgical menopause.”
It is used after menopause for women, when the estrogen levels begin dropping, some women get very uncomfortable symptoms, such as feelings of warmth in the face, neck, and chest, or sudden intense episodes of heat and sweating (“hot flashes” or “hot flushes”). In some women, the symptoms are mild, and they will not need to take medicines. In other women, symptoms can be more severe, help reduce your chances of developing osteoporosis (thin, weak bones)
If you use Duavee only to prevent osteoporosis due to menopause, talk with your healthcare provider about whether a different treatment or medicine without estrogens might be better for you.
Duavee should be taken for the shortest time possible and only for as long as treatment is needed.
You and your healthcare provider should talk regularly about whether you still need treatment with Duavee.
Duavee is not for use in children.
It is not known if Duavee is safe and effective in people with kidney problems.
Description
DUAVEE (conjugated estrogens/bazedoxifene), contains conjugated estrogens with bazedoxifene, an estrogen agonist/antagonist.
Conjugated estrogens are purified from pregnant mares’ urine and consist of the sodium salts of water-soluble estrogen sulfates blended to represent the average composition of material derived from pregnant mares’ urine. Conjugated estrogens are a mixture of sodium estrone sulfate and sodium equilin sulfate, and also contain as concomitant components, sodium sulfate conjugates, 17α-dihydroequilin, 17α-estradiol, and 17β-dihydroequilin.
Bazedoxifene is supplied as the acetate salt (bazedoxifene acetate) and has the chemical name 1H-Indol-5-ol, 1-[[4-[2-(hexahydro-1H-azepin-1-yl) ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methyl-, monoacetate. The empirical formula is C30H34N2O3 ∙ C2H4O2, and the molecular weight is 530.65.
Bazedoxifene acetate is a white to tan powder. The aqueous solubility of bazedoxifene is pH-dependent. Solubility is higher at lower pH. The solubility of bazedoxifene acetate in unbuffered sterile water was measured to be 923 microgramsA/mL at pH 5.4. The following represents the chemical structure of bazedoxifene acetate:
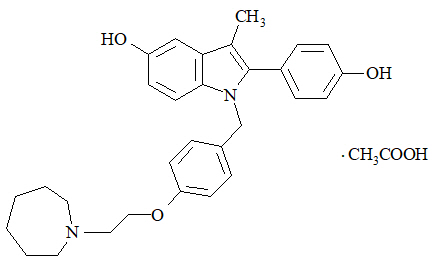
DUAVEE is available for oral administration as tablets containing 0.45 mg of conjugated estrogens with 20 mg of bazedoxifene (equivalent to 22.6 mg of bazedoxifene acetate). Each tablet of DUAVEE contains the following inactive ingredients: calcium phosphate tribasic, hydroxypropyl cellulose, microcrystalline cellulose, powdered cellulose, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, sucrose, ascorbic acid, sucrose palmitic acid ester, hydroxyethylcellulose, titanium dioxide, red iron oxide, yellow iron oxide, black iron oxide, povidone, polydextrose, maltitol, poloxamer 188, propylene glycol, and isopropyl alcohol.
Mechanism of Action
DUAVEE pairs conjugated estrogens with bazedoxifene. Conjugated estrogens and bazedoxifene function by binding to and activating estrogen receptors (ER) α and β, which vary in proportion from tissue to tissue. Conjugated estrogens are composed of multiple estrogens and are agonists of ER- α and β. Bazedoxifene is an estrogen agonist/antagonist that acts as an agonist in some estrogen-sensitive tissues and an antagonist in others (e.g., uterus). The pairing of conjugated estrogens with bazedoxifene produces a composite effect that is specific to each target tissue. The bazedoxifene component reduces the risk of endometrial hyperplasia that can occur with the conjugated estrogens component.
Pharmacodynamic studies have not been conducted with DUAVEE.
What is the most important information I should know about Duavee?
- Do not take additional estrogen products while you are taking Duavee.
- Using estrogens may increase your chance of getting cancer of the uterus (womb).
- Report any unusual vaginal bleeding right away while you are taking Duavee. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
- Do not use estrogens to prevent heart disease, heart attacks, strokes or dementia (decline in brain function).
- Using estrogens may increase your chances of getting strokes or blood clots.
- Using estrogens may increase your chance of getting dementia, based on a study of women 65 years of age or older.
- You and your healthcare provider should talk regularly about whether you still need treatment with Duavee.
Who should not take Duavee?
Do not take Duavee if you:
- currently have or have had blood clots
- are allergic to estrogens or bazedoxifene, the active ingredients in Duavee, or any of its ingredients.
See the list of ingredients in Duavee at the end of this leaflet. - have unusual vaginal bleeding. Vaginal bleeding after menopause may be a warning sign of cancer of the uterus (womb). Your healthcare provider should check any unusual vaginal bleeding to find out the cause.
- currently have or have had certain cancers. Estrogens may increase the chances of getting certain types of cancers, including cancer of the breast or uterus. If you have or have had cancer, talk with your healthcare provider about whether you should use Duavee.
- currently have or have had liver problems
- have been diagnosed with a bleeding disorder
- are pregnant. Duavee is not for pregnant women. If you think you may be pregnant, you should have a pregnancy test and know the results. Do not take Duavee if the test is positive and talk to your healthcare provider.
What should I tell my healthcare provider before taking Duavee?
Before you take Duavee, tell your healthcare provider if you:
- have any unusual vaginal bleeding.
- have any other medical conditions. Your healthcare provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing), epilepsy (seizures), diabetes, migraine, endometriosis, lupus, or problems with your heart, liver, thyroid, kidneys, or have high calcium levels in your blood.
- are going to have surgery or will be on bed rest. Your healthcare provider will let you know if you need to stop taking Duavee.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take other hormonal medicines, including progestins or other medicines like Duavee. Ask your healthcare provider if you do not know if you take any of these medicines.
Some medicines may affect how Duavee works. Duavee may also affect how your other medicines work. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take Duavee?
- Duavee comes in a blister package.
- Record the date you open the foil pouch in the space provided on the blister package label. Do not use if the blister package has been open for more than 60 days.
- Take Duavee exactly as your healthcare provider tells you to take it.
- Take 1 Duavee tablet at the same time each day.
- Duavee should be swallowed whole.
- Take Duavee with or without food.
- You should not remove Duavee from the blister until right before you are ready to take it. Remove 1 tablet at a time from the blister package. Do not place Duavee in pill boxes or pill organizers.
- If you miss a dose of Duavee, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Take the next dose at your regular time. Do not take 2 doses at the same time unless your healthcare provider tells you to. If you are not sure about your dosing, call your healthcare provider.
- If you take a calcium or vitamin D supplement, you may take it at the same time you take Duavee.
- If you take too much Duavee, call your healthcare provider. Symptoms of taking too much Duavee include:
What are the possible side effects of Duavee?
Side effects are grouped by how serious they are and how often they happen when you are treated.
Serious side effects include:
- blood clots
- stroke
- heart attack
- cancer of the lining of the uterus
- breast cancer
- cancer of the ovary
- dementia
- gallbladder problems
- loss of vision
- high blood pressure
- increased fats in your blood
- liver problems
- thyroid problems
- fluid retention
- low calcium
- swelling of your mouth or tongue
- worsening of other medical problems such as asthma, diabetes, epilepsy, migraines, a genetic problem called porphyria, lupus and liver problems
Call your healthcare provider right away if you get any of the following warning signs, or any other unusual symptoms that concern you:
- new breast lumps
- unusual vaginal bleeding
- changes in vision or speech
- sudden new severe headaches
- severe pains in your chest or legs with or without shortness of breath, weakness and fatigue
Less serious, but common side effects include:
These are not all the possible side effects of Duavee. For more information, ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effects that bother you or do not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What can I do to lower my chances of a serious side effect with Duavee?
- Talk with your healthcare provider regularly about whether you should continue taking Duavee.
- See you healthcare provider right away if you get vaginal bleeding while taking Duavee.
- Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your healthcare provider tells you something else.
- If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often.
- If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have higher chances of getting heart disease.
Ask your healthcare provider for ways to lower your chances of getting heart disease.
General information about the safe and effective use of Duavee
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Duavee for a condition for which it was not prescribed. Do not give Duavee to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information summarizes the most important information about Duavee. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Duavee that is written for health professionals.
For more information, go to www.DUAVEE.com, or call 1-800-438-1985.
How should I store Duavee?
- Store Duavee at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Duavee in the blister until you are ready to take it to protect the tablet from moisture.
- Do not place Duavee in pill boxes or pill organizers.
- After opening the foil pouch the Duavee blisters come in, Duavee must be used within 60 days.
Keep Duavee and all other medicines out of the reach of children.
What are the ingredients in Duavee?
Active Ingredients: conjugated estrogens and bazedoxifene. Conjugated estrogens are a mixture of sodium estrone sulfate and sodium equilin sulfate and other components, including sodium sulfate conjugates, 17α-dihydroequilin, 17α-estradiol, and 17β-dihydroequilin.
Inactive Ingredients: calcium phosphate tribasic, hydroxypropyl cellulose, microcrystalline cellulose, powdered cellulose, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, sucrose, ascorbic acid, sucrose palmitic acid ester, hydroxyethylcellulose, titanium dioxide, red iron oxide, yellow iron oxide, black iron oxide, povidone, polydextrose, maltitol, poloxamer 188, propylene glycol, isopropyl alcohol.
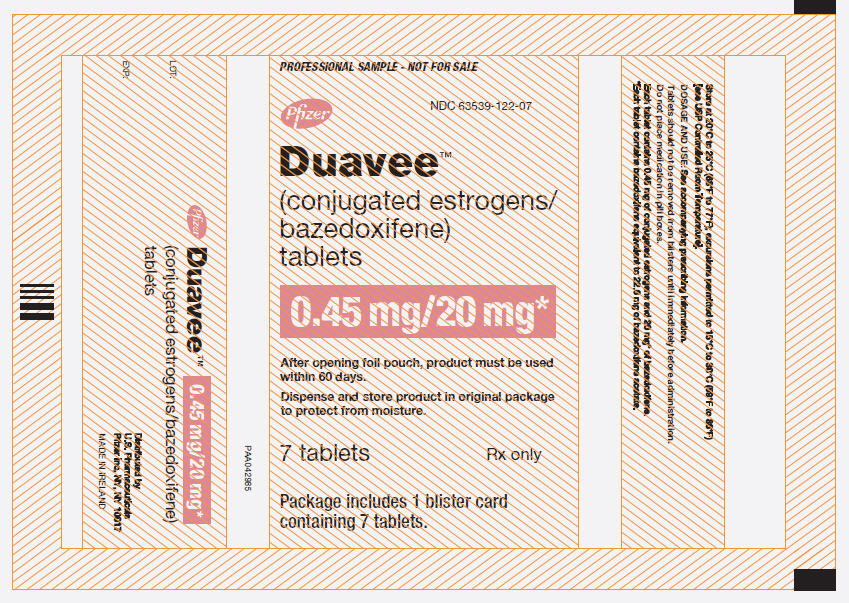
Label
PRINCIPAL DISPLAY PANEL – 7 TABLET BLISTER POUCH
- NDC 63539-122-07
- Pfizer
- Duavee™
- (conjugated estrogens/
bazedoxifene)
tablets - 0.45 mg/20 mg*
- After opening foil pouch, product must be used
within 60 days. - Dispense and store product in original package
to protect from moisture. - 7 tablets
Rx only - Package includes 1 blister card
containing 7 tablets.
SRC: NLM .
