Droxia
Generic name: hydroxyurea
Brand names: Droxia, Hydrea, Mylocel, Siklos
Drug class: Antimetabolites
Medically reviewed by A Ras MD.
What is Droxia?
Droxia is a prescription medicine that is used to reduce the frequency of painful crises and reduce the need for blood transfusions in people with sickle cell anemia with recurrent moderate to severe painful crises.
It is not known if Droxia is safe and effective in children.
Description
DROXIA® (hydroxyurea capsules, USP) is available for oral use as capsules containing 200 mg, 300 mg, and 400 mg hydroxyurea. Inactive ingredients include citric acid, gelatin, lactose, magnesium stearate, sodium phosphate, titanium dioxide, and capsule colorants: FD&C Blue No. 1 and FD&C Green No. 3 (200 mg capsules); D&C Red No. 28, D&C Red No. 33, and FD&C Blue No. 1 (300 mg capsules); D&C Red No. 28, D&C Red No. 33, and D&C Yellow No. 10 (400 mg capsules).
Hydroxyurea is a white to off-white crystalline powder. It is hygroscopic and freely soluble in water, but practically insoluble in alcohol. The empirical formula is CH4N2O2 and it has a molecular weight of 76.05. Its structural formula is:
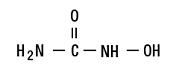
Mechanism of Action
The precise mechanism by which hydroxyurea produces its cytotoxic and cytoreductive effects is not known. However, various studies support the hypothesis that hydroxyurea causes an immediate inhibition of DNA synthesis by acting as a ribonucleotide reductase inhibitor, without interfering with the synthesis of ribonucleic acid or of protein.
The mechanisms by which DROXIA produces its beneficial effects in patients with sickle cell anemia (SCA) are uncertain. Known pharmacologic effects of DROXIA that may contribute to its beneficial effects include increasing hemoglobin F levels in red blood cells (RBCs), decreasing neutrophils, increasing the water content of RBCs, increasing deformability of sickled cells, and altering the adhesion of RBCs to endothelium.
What is the most important information I should know about Droxia?
Droxia can cause serious side effects including:
- Low blood counts are common with Droxia, including low red blood cells, white blood cells, and platelets, and can be severe and life-threatening. If your white blood cell count becomes very low, you are at increased risk for infection. Your healthcare provider will check your blood cell counts before and during treatment with Droxia. Your healthcare provider may change your dose or tell you to stop taking Droxia if you have low blood cell counts. Tell your healthcare provider right away if you get any of the following symptoms:
- Fever
- feeling very tired
- chills
- shortness of breath
- body aches
- bleeding or unexplained bruising
- Cancer. Some people have developed cancer, such as leukemia and skin cancer, after taking Droxia for a long time. Your healthcare provider will check you for cancer. You should protect your skin from the sun using sunblock, hats, and sun-protective clothing.
- Droxia can harm your unborn baby.
For females taking Droxia who can become pregnant:
- You should talk with your healthcare provider about the risks of Droxia to your unborn baby.
- You should use effective birth control during treatment with Droxia and for at least 6 months after treatment.
- Your healthcare provider will perform a pregnancy test before you start treatment with Droxia.
- You should avoid becoming pregnant during treatment with Droxia. Tell your healthcare provider right away if you become pregnant or think you may be pregnant.
For males taking Droxia:
- Droxia can affect your sperm. If you have a female sexual partner who can become pregnant, you should use a condom during treatment with Droxia and for at least 1 year after treatment.
Droxia may cause fertility problems in males. Talk to your healthcare provider if this is a concern for you.
See “What are the possible side effects of Droxia?” for more information about side effects.
Who should not take Droxia?
Do not take Droxia if you are allergic to hydroxyurea or any of the ingredients in Droxia. See the end of this leaflet for a list of the ingredients in Droxia.
What should I tell my healthcare provider before taking Droxia?
Before taking Droxia, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems or are receiving hemodialysis.
- have human immunodeficiency virus (HIV). Taking Droxia with certain HIV medicines can cause serious reactions and may lead to death. Tell your healthcare provider if you take an HIV medicine.
- have increased level of uric acid in your blood (hyperuricemia).
- have a history of receiving interferon therapy or are currently receiving interferon therapy.
- have leg wounds or ulcers
- plan to receive any vaccinations. You should not receive “live vaccines” during treatment with Droxia.
- are pregnant or plan to become pregnant. See “What is the most important information I should know about Droxia?”
- are breastfeeding or plan to breastfeed. Droxia can pass into your breast milk. Do not breastfeed during treatment with Droxia.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Droxia?
- Take Droxia exactly as your healthcare provider tells you. Droxia is taken 1 time a day.
- If you take too much Droxia, call your healthcare provider or go to the nearest hospital emergency room right away.
- Droxia capsules must be handled with care. To decrease the risk of exposure, you or your caregivers should do the following when handling Droxia:
- Wear disposable gloves when handling Droxia capsules or bottles containing Droxia.
- Wash your hands with soap and water before and after handling DROXIA capsules or bottles containing Droxia.
- Do not open, break, or chew the capsules.
- Avoid contact with crushed or opened capsules. If contact from crushed or opened capsules happens on the skin, wash the skin area right away and thoroughly with soap and water. If contact from crushed or opened capsules happens in the eyes, flush the eyes thoroughly with water or isotonic eyewash used for that purpose for at least 15 minutes.
- If the powder from the capsule is spilled, wipe it up right away with a damp disposable towel. Throw the damp disposable towel and the empty capsules away in a closed container, such as a plastic bag. The spill areas should then be cleaned three times using a detergent solution followed by clean water.
What are the possible side effects of Droxia?
Droxia may cause serious side effects, including:
- See “What is the most important information I should know about Droxia?”
- Skin ulcers and death of tissue (gangrene) have happened in people who take Droxia. This has happened most often in people who receive interferon therapy or have a history of interferon therapy. Your healthcare provider will stop treatment with Droxia if you develop any skin ulcers.
- Enlarged red blood cells (macrocytosis). Macrocytosis is common in people who take Droxia and can make it difficult to detect a decrease of folic acid. Your healthcare provider may prescribe a folic acid supplement for you.
- Respiratory (breathing) problems. Some people have developed life-threatening respiratory conditions called interstitial lung disease. Your healthcare provider may tell you to stop taking Droxia if you develop respiratory problems. Tell your healthcare provider right away if you get any of the following symptoms:
- fever
- shortness of breath
- cough
The most common side effects of Droxia include:
- reduced blood counts
- bleeding
- mouth ulcers
- nausea
- diarrhea or constipation
- loss of appetite
These are not all the possible side effects of Droxia.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Droxia
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Droxia for a condition for which it was not prescribed. Do not give Droxia to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Droxia that is written for health professionals.
How should I store Droxia?
- Store Droxia at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep the Droxia bottle tightly closed.
- Call your healthcare provider for instructions on how to throw away (dispose of) Droxia that is out of date.
Keep Droxia and all medicines out of the reach of children. Keep Droxia away from pets.
What are the ingredients in Droxia?
Active ingredient: hydroxyurea
Inactive ingredients: citric acid, gelatin, lactose, magnesium stearate, sodium phosphate, titanium dioxide, and capsule colorants: FD&C Blue No. 1 and FD&C Green No. 3 (200 mg capsules); D&C Red No. 28, D&C Red No. 33, and FD&C Blue No. 1 (300 mg capsules); D&C Red No. 28, D&C Red No. 33, and D&C Yellow No. 10 (400 mg capsules).
Label
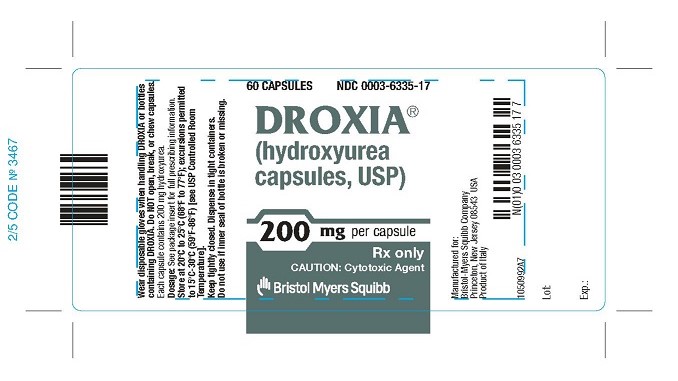
DROXIA 200 MG CAPSULES REPRESENTATIVE PACKAGING
- See How Supplied section for a complete list of available packages of DROXIA.
- 60 CAPSULES
NDC 0003-6335-17
DROXIA®
(hydroxyurea capsules, USP)
200 mg per capsule
Rx only
CAUTION: Cytotoxic Agent - Bristol-Myers Squibb
- 60 CAPSULES
NDC 0003-6335-17
DROXIA®
(hydroxyurea capsules, USP)
200 mg per capsule
Rx only
CAUTION: Cytotoxic Agent - Bristol-Myers Squibb


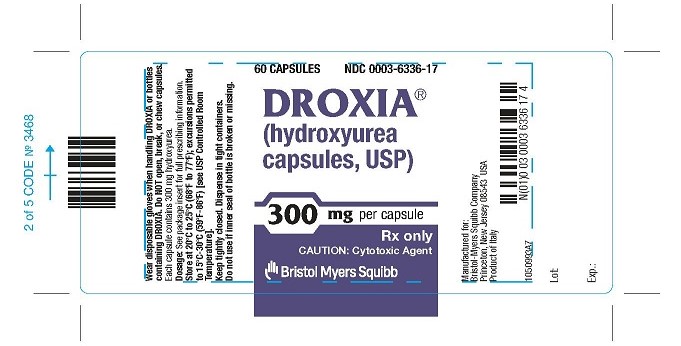
DROXIA 300 MG CAPSULES REPRESENTATIVE PACKAGING
- 60 CAPSULES
NDC 0003-6336-17
DROXIA®
(hydroxyurea capsules, USP)
300 mg per capsule
Rx only
CAUTION: Cytotoxic Agent - Bristol-Myers Squibb
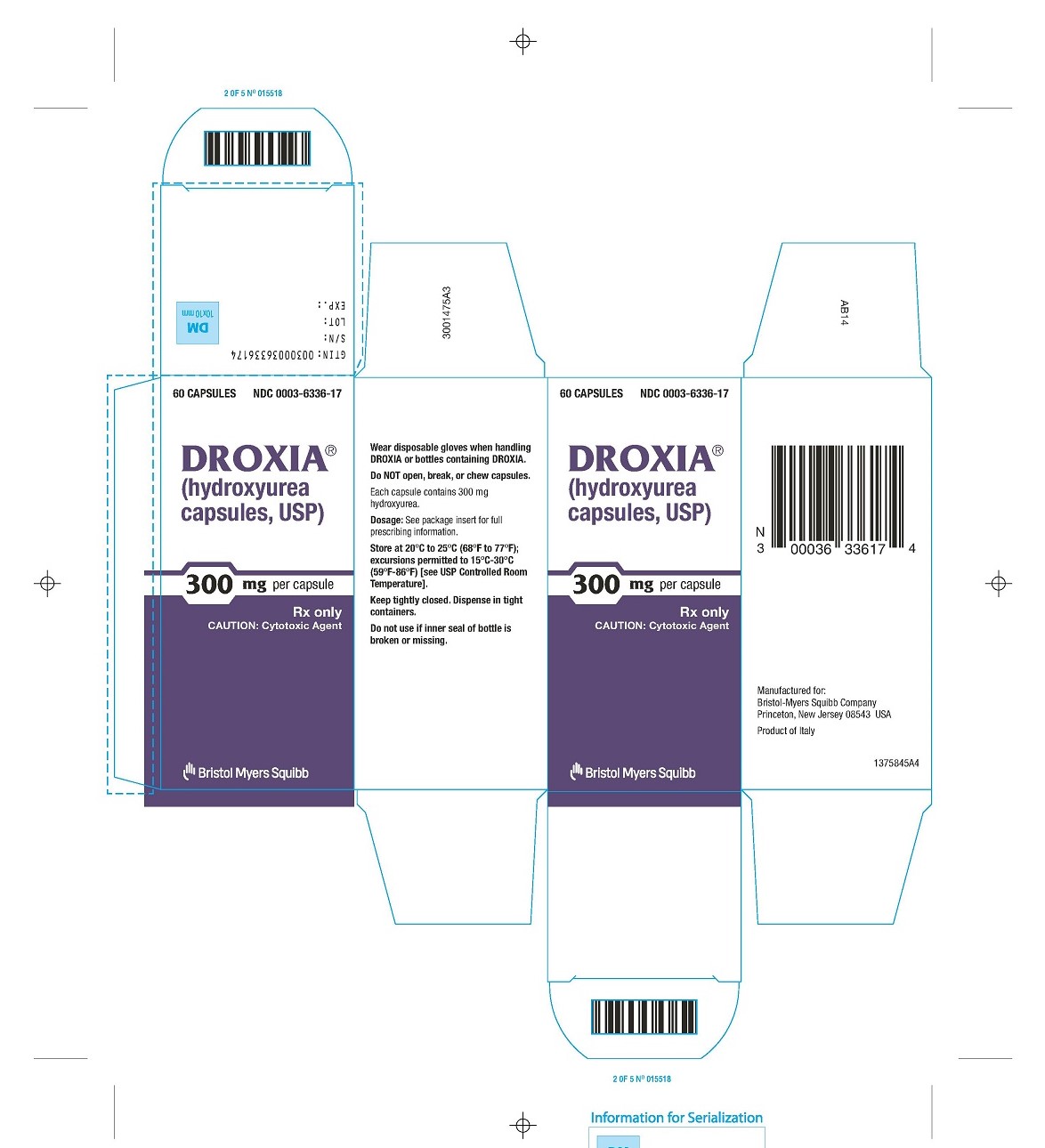
- 60 CAPSULES
NDC 0003-6336-17
DROXIA®
(hydroxyurea capsules, USP)
300 mg per capsule
Rx only
CAUTION: Cytotoxic Agent - Bristol-Myers Squibb
DROXIA 400 MG CAPSULES REPRESENTATIVE PACKAGING
- 60 CAPSULES
NDC 0003-6337-17
DROXIA®
(hydroxyurea capsules, USP)
400 mg per capsule
Rx only
CAUTION: Cytotoxic Agent - Bristol-Myers Squibb
- 60 CAPSULES
NDC 0003-6337-17
DROXIA®
(hydroxyurea capsules, USP)
400 mg per capsule
Rx only
CAUTION: Cytotoxic Agent - Bristol-Myers Squibb


SRC: NLM .

