Detrol LA
Generic name: tolterodine
Brand names: Detrol, Detrol LA
Drug class: Urinary antispasmodics
Medically reviewed by A Ras MD.
What is Detrol LA?
Detrol LA is a prescription medicine for adults used to treat the following symptoms due to a condition called overactive bladder Having a strong need to urinate with leaking or wetting accidents (urge urinary incontinence), Having a strong need to urinate right away (urgency), Having to urinate often (frequency).
Detrol LA did not help the symptoms of overactive bladder when studied in children.
Description
DETROL LA Capsules contain tolterodine tartrate. The active moiety, tolterodine, is a muscarinic receptor antagonist. The chemical name of tolterodine tartrate is (R)-N,N-diisopropyl-3-(2-hydroxy-5-methylphenyl)-3-phenylpropanamine L-hydrogen tartrate. The empirical formula of tolterodine tartrate is C26H37NO7,. Its structure is:

Tolterodine tartrate is a white, crystalline powder with a molecular weight of 475.6. The pKa value is 9.87 and the solubility in water is 12 mg/mL. It is soluble in methanol, slightly soluble in ethanol, and practically insoluble in toluene. The partition coefficient (Log D) between n-octanol and water is 1.83 at pH 7.3.
DETROL LA 4 mg capsule for oral administration contains 4 mg of tolterodine tartrate. Inactive ingredients are sucrose, starch, hypromellose, ethylcellulose, medium chain triglycerides, oleic acid, gelatin, and FD&C Blue #2.
DETROL LA 2 mg capsule for oral administration contains 2 mg of tolterodine tartrate, and the following inactive ingredients: sucrose, starch, hypromellose, ethylcellulose, medium chain triglycerides, oleic acid, gelatin, yellow iron oxide, and FD&C Blue #2.
Both the 2 mg and 4 mg capsule strengths are imprinted with a pharmaceutical grade printing ink that contains shellac glaze, titanium dioxide, propylene glycol, and simethicone.
Mechanism of Action
Tolterodine acts as a competitive antagonist of acetylcholine at postganglionic muscarinic receptors. Both urinary bladder contraction and salivation are mediated via cholinergic muscarinic receptors.
After oral administration, tolterodine is metabolized in the liver, resulting in the formation of 5-hydroxymethyl tolterodine (5-HMT), the major pharmacologically active metabolite. 5-HMT, which exhibits an antimuscarinic activity similar to that of tolterodine, contributes significantly to the therapeutic effect. Both tolterodine and 5-HMT exhibit a high specificity for muscarinic receptors, since both show negligible activity or affinity for other neurotransmitter receptors and other potential cellular targets, such as calcium channels.
Who should not take Detrol LA?
Do not take Detrol LA if:
- You have trouble emptying your bladder (also called “urinary retention”).
- Your stomach empties slowly (also called “gastric retention”).
- You have an eye problem called “uncontrolled narrow-angle glaucoma”.
- You are allergic to Detrol LA or to any of its ingredients. See the end of this leaflet for a complete list of ingredients.
- You are allergic to Toviaz, which contains fesoterodine.
What should I tell my healthcare provider before taking Detrol LA?
Before starting Detrol LA, tell your doctor about all of your medical conditions, including if you:
- Have any stomach or intestinal problems.
- Have trouble emptying your bladder or you have a weak urine stream.
- Have an eye problem called narrow-angle glaucoma.
- Have liver problems.
- Have kidney problems.
- Have a condition called myasthenia gravis.
- Or any family members have a rare heart condition called QT prolongation (long QT syndrome).
- Are pregnant or trying to become pregnant. It is not known if Detrol LA could harm your unborn baby.
- Are breastfeeding. It is not known if Detrol LA passes into your milk and if it can harm your child.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Other drugs can affect how your body handles DETROL LA. Your doctor may use a lower dose of Detrol LA if you are taking:
- Certain medicines for fungus or yeast infections such as Nizoral (ketoconazole), Sporanox® (itraconazole), or Monistat (miconazole).
- Certain medicines for bacteria infections such as Biaxin (clarithromycin).
- Certain medicines for treatment of HIV infection such as Norvir (ritonavir), Invirase® (saquinavir), Reyataz (atazanavir).
- Sandimmune (cyclosporine) or Velban (vinblastine).
Know the medicines you take. Keep a list of them with you to show your doctor or pharmacist each time you get a new medicine.
How should I take Detrol LA?
- Take Detrol LA exactly as prescribed. Your doctor will prescribe the dose that is right for you. Do not change your dose unless told to do so by your doctor.
- Take Detrol LA capsules once a day with liquid. Swallow the whole capsule. Tell your doctor if you cannot swallow a capsule.
- Detrol LA can be taken with or without food.
- Take Detrol LA the same time each day.
- If you miss a dose of Detrol LA, begin taking Detrol LA again the next day. Do not take 2 doses of Detrol LA in the same day.
- If you took more than your prescribed dose of Detrol LA, call your doctor or poison control center, or go to the hospital emergency room.
What are the possible side effects of Detrol LA?
Detrol LA may cause allergic reactions that may be serious. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat, or tongue. If you experience these symptoms, you should stop taking Detrol LA and get emergency medical help right away.
The most common side effects with Detrol LA are:
- Dry mouth
- Headache
- Constipation
- Stomach pain
Medicines like Detrol LA can cause blurred vision, dizziness, and drowsiness.
Do not drive, operate machinery, or do other dangerous activities until you know how DETROL LA affects you.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
These are not all the side effects with Detrol LA. For a complete list, ask your doctor or pharmacist.
General information about the safe and effective use of Detrol LA
Medicines are sometimes prescribed for conditions that are not in the patient information leaflet. Only use Detrol LA the way your doctor tells you. Do not share it with other people even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about Detrol LA. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Detrol LA that is written for health professionals.
How should I store Detrol LA?
- Store Detrol LA at room temperature, 68° – 77°F (20° – 25°C); brief periods permitted between 59° – 86°F (15° – 30°C). Protect from light. Keep in a dry place.
- Keep Detrol LA and all medicines out of the reach of children.
What are the ingredients in Detrol LA?
Active ingredients: tolterodine tartrate
Inactive ingredients: sucrose, starch, hypromellose, ethylcellulose, medium chain triglycerides, oleic acid, gelatin, and FD&C Blue #2. 2 mg capsule also contains yellow iron oxide. Capsules have pharmaceutical grade printing ink that contains shellac glaze, titanium dioxide, propylene glycol, and simethicone.
Label
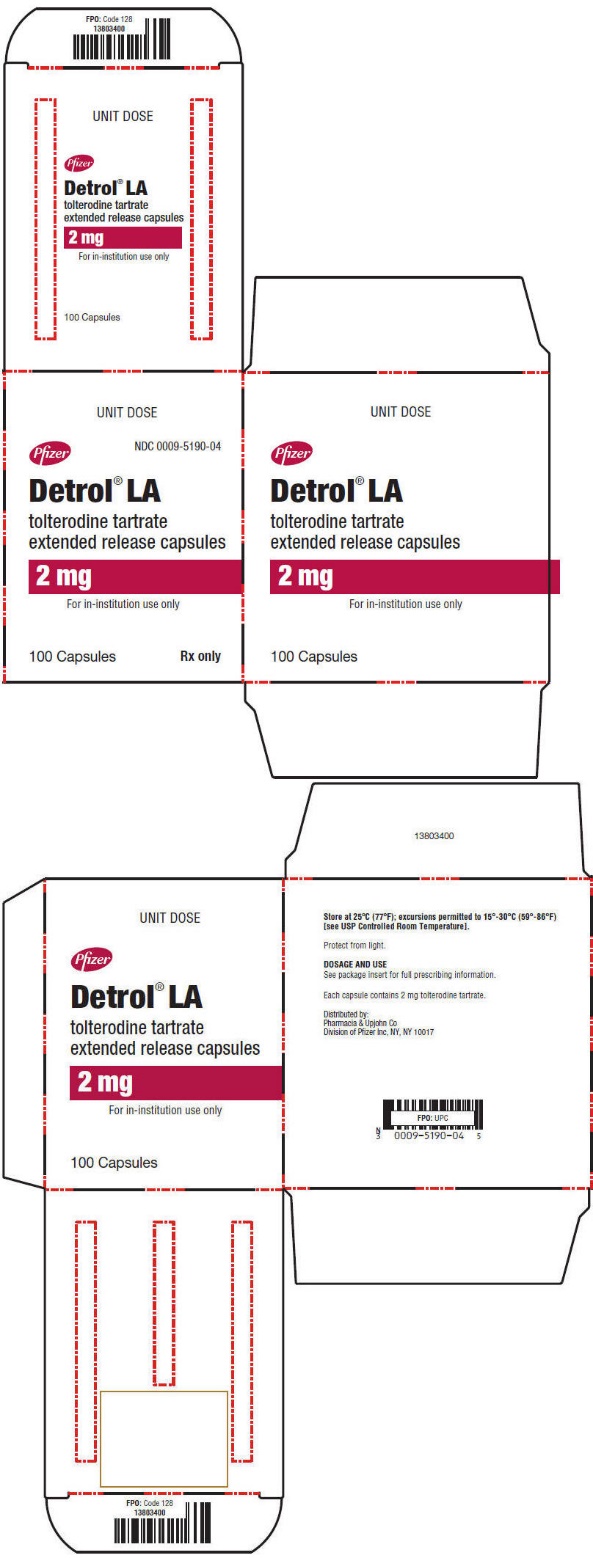
PRINCIPAL DISPLAY PANEL – 2 mg Capsule Unit Dose Carton
- UNIT DOSE
- Pfizer
NDC 0009-5190-04 - Detrol® LA
tolterodine tartrate
extended release capsules - 2 mg
- For in-institution use only
- 100 Capsules
Rx only
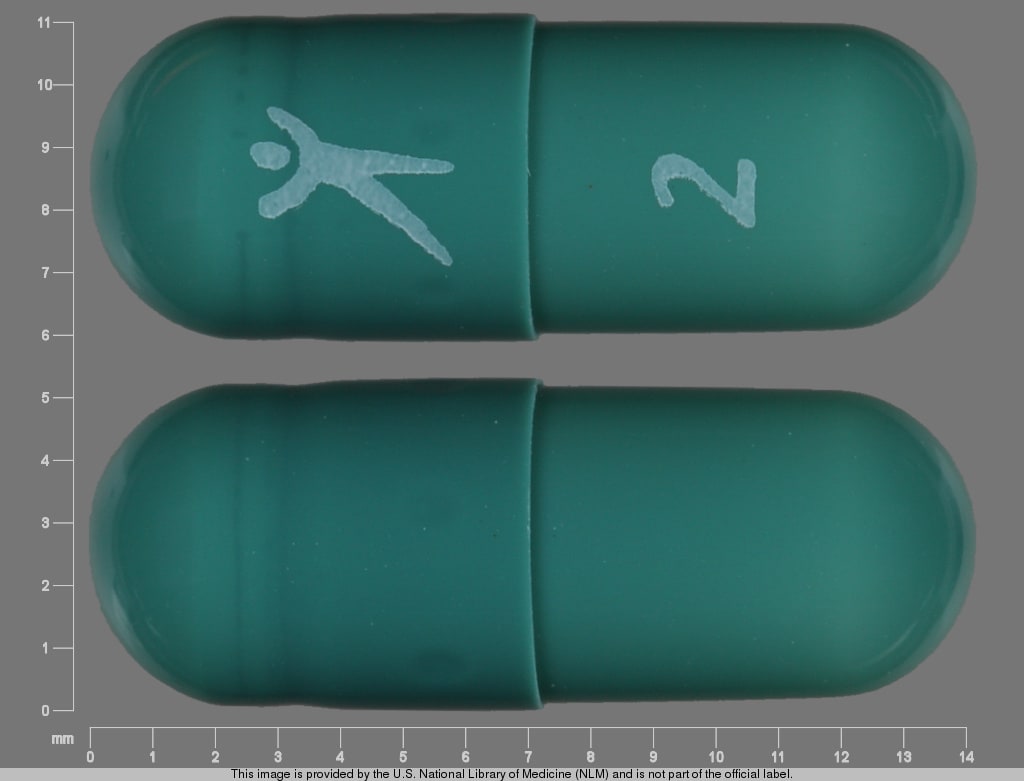
PRINCIPAL DISPLAY PANEL – 30 Capsule 4 mg Bottle Label
- NDC 0009-5191-01
- 30 Capsules
Rx only - Detrol® LA
tolterodine tartrate
extended release capsules - 4 mg
- Pfizer
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017


SRC: NLM .
