Delstrigo
Generic name: doravirine, lamivudine, and tenofovir disoproxil fumarate
Drug class: Antiviral combinations
Medically reviewed by A Ras MD.
What is Delstrigo?
Delstrigo is a prescription medicine that is used without other HIV-1 medicines to treat HIV-1 infection in adults who have not received HIV-1 medicines in the past or to replace their current HIV-1 medicines for people whose healthcare provider determines that they meet certain requirements.
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS). Delstrigo contains the prescription medicines doravirine, lamivudine and tenofovir disoproxil fumarate. It is not known if Delstrigo is safe and effective in children under 18 years of age.
Description
DELSTRIGO is a fixed-dose combination, film-coated tablet, containing doravirine, lamivudine, and TDF for oral administration.
Doravirine is an HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI).
Lamivudine is the (-)enantiomer of a dideoxy analogue of cytidine and is an HIV-1 nucleoside analogue reverse transcriptase inhibitor.
TDF (a prodrug of tenofovir) is a fumaric acid salt of the bis-isopropoxycarbonyloxymethyl ester derivative of tenofovir. In vivo TDF is converted to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate. Tenofovir is an HIV-1 reverse transcriptase inhibitor.
Each tablet contains 100 mg of doravirine, 300 mg of lamivudine, and 300 mg of TDF (equivalent to 245 mg of tenofovir disoproxil) as active ingredients. The tablets include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and sodium stearyl fumarate. The tablets are film coated with a coating material containing the following inactive ingredients: hypromellose, iron oxide yellow, lactose monohydrate, titanium dioxide, and triacetin. The coated tablets are polished with carnauba wax.
Doravirine:
The chemical name for doravirine is 3-chloro-5-[[1-[(4,5-dihydro-4-methyl-5-oxo-1H-1,2,4-triazol-3-yl)methyl]-1,2-dihydro-2-oxo-4-(trifluoromethyl)-3-pyridinyl]oxy]benzonitrile.
It has a molecular formula of C17H11ClF3N5O3 and a molecular weight of 425.75.
It has the following structural formula:
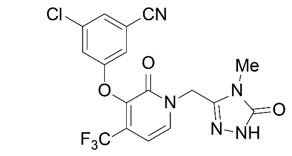
Doravirine is practically insoluble in water.
Lamivudine:
The chemical name for lamivudine is (-)-1-[(2R,5S)-2-(hydroxymethyl)-1,3-oxathiolan-5-yl]-cytosine.
It has a molecular formula of C8H11N3O3S and a molecular weight of 229.26.
It has the following structural formula:

Lamivudine is soluble in water.
TDF:
The chemical name for TDF is 9-[(R)-2-[[bis[[(isopropoxycarbonyl)oxy]methoxy] phosphinyl]- methoxy]propyl]adenine fumarate (1:1).
It has a molecular formula of C19H30N5O10 P∙C4H4O4 and a molecular weight of 635.52.
It has the following structural formula:
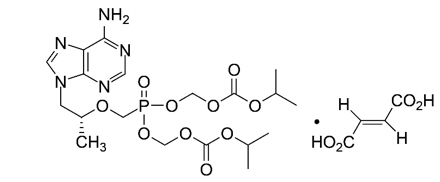
TDF is slightly soluble in water.
What is the most important information I should know about Delstrigo?
Delstrigo can cause serious side effects, including:
Worsening of hepatitis B virus infection (HBV). If you have Human Immunodeficiency Virus-1 (HIV-1) and HBV infection, your HBV infection may get worse (flare-up) if you stop taking Delstrigo. A “flare-up” is when your HBV infection suddenly returns in a worse way than before. Your doctor will test you for HBV infection before you start treatment with Delstrigo.
- Do not run out of Delstrigo. Refill your prescription or talk to your doctor before your Delstrigo is all gone.
- Do not stop taking Delstrigo without first talking to your doctor. If you stop taking Delstrigo, your doctor will need to check your health often and do blood tests regularly for several months to check your liver. Tell your doctor about any new or unusual symptoms you may have after you stop taking Delstrigo.
For more information about side effects, see “What are the possible side effects of Delstrigo?”
Who should not take Delstrigo?
Do not take Delstrigo if you take any of the following medicines:
- carbamazepine
- oxcarbazepine
- phenobarbital
- phenytoin
- enzalutamide
- rifampin
- rifapentine
- mitotane
- St. John’s wort
Ask your doctor or pharmacist if you are not sure if your medicine is one that is listed above. If you have taken any of the medicines in the past 4 weeks, talk to your doctor or pharmacist before starting treatment with Delstrigo.
Do not take Delstrigo if you have ever had an allergic reaction to lamivudine.
What should I tell my healthcare provider before taking Delstrigo?
Before treatment with Delstrigo, tell your doctor about all of your medical conditions, including if you:
- have hepatitis B virus infection
- have kidney problems
- have bone problems, including a history of bone fractures
- are pregnant or plan to become pregnant. It is not known if Delstrigo can harm your unborn baby. Tell your doctor if you become pregnant during treatment with Delstrigo.
Pregnancy Registry: There is a pregnancy registry for people who take Delstrigo during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your doctor about how you can take part in this registry. - are breastfeeding or plan to breastfeed. Do not breastfeed if you take Delstrigo.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- Two of the medicines in Delstrigo (lamivudine and tenofovir) can pass into your breast milk. It is not known if doravirine can pass into your breast milk.
- Talk with your doctor about the best way to feed your baby.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
- Some medicines interact with Delstrigo. Keep a list of your medicines to show your doctor and pharmacist.
- Tell your doctor if you have taken rifabutin in the past 4 weeks.
- You can ask your doctor or pharmacist for a list of medicines that interact with Delstrigo.
- Do not start taking a new medicine without telling your doctor. Your doctor can tell you if it is safe to take Delstrigo with other medicines.
How should I take Delstrigo?
- Take Delstrigo every day exactly as your doctor tells you to take it.
- Take Delstrigo 1 time each day, at about the same time every day.
- Delstrigo is usually taken by itself (without other HIV-1 medicines).
- If you take the medicine rifabutin during treatment with Delstrigo, your doctor will also prescribe an additional dose of doravirine for you. You may not have enough doravirine in your blood if you take rifabutin during treatment with Delstrigo. Carefully follow your doctor’s instructions about when to take doravirine and how much to take. This is usually 1 tablet of doravirine about 12 hours after your last dose of Delstrigo.
- Take Delstrigo with or without food.
- Do not change your dose or stop taking Delstrigo without talking to your doctor. Stay under a doctor’s care when taking Delstrigo.
- It is important that you do not miss or skip doses of Delstrigo.
- If you miss a dose of Delstrigo, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose and take the next dose at your regular time. Do not take 2 doses of Delstrigo at the same time.
- If you have any questions, call your doctor or pharmacist.
- If you take too much Delstrigo, call your doctor or go to the nearest hospital emergency room right away.
- When your Delstrigo supply starts to run low, get more from your doctor or pharmacy. This is very important because the amount of virus in your blood may increase if the medicine is stopped for even a short time. The virus may develop resistance to Delstrigo and become harder to treat.
What are the possible side effects of Delstrigo?
Delstrigo may cause serious side effects, including:
- See “What is the most important information I should know about Delstrigo?”
- New or worse kidney problems, including kidney failure. Your doctor should do blood and urine tests to check your kidneys before you start and during treatment with Delstrigo. Your doctor may tell you to stop taking Delstrigo if you develop new or worse kidney problems.
- Bone problems can happen in some people who take Delstrigo. Bone problems include bone pain, softening or thinning (which may lead to fractures). Your doctor may need to do tests to check your bones.
Tell your doctor if you have any of the following symptoms during treatment with Delstrigo: bone pain that does not go away or worsening bone pain, pain in your arms, legs, hands or feet, broken (fractured) bones or muscle pain or weakness. These may be symptoms of a bone or kidney problem.
- Changes in your immune system (Immune Reconstitution Syndrome) can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your doctor right away if you start having any new symptoms after starting your HIV-1 medicine.
The most common side effects of Delstrigo include dizziness, nausea, and abnormal dreams.
These are not all the possible side effects of Delstrigo.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Delstrigo
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use Delstrigo for a condition for which it was not prescribed. Do not give Delstrigo to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about Delstrigo that is written for healthcare professionals.
How should I store Delstrigo?
- Store Delstrigo tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Delstrigo in the original bottle.
- Do not take the tablets out of the bottle to store in another container, such as a pill box.
- Keep the bottle tightly closed to protect Delstrigo from moisture.
- The Delstrigo bottle contains desiccants to help keep your medicine dry (protect it from moisture). Keep the desiccants in the bottle. Do not eat the desiccants.
Keep Delstrigo and all medicines out of the reach of children.
What are the ingredients in Delstrigo?
Active ingredients: doravirine, lamivudine, and tenofovir disoproxil fumarate.
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and sodium stearyl fumarate. The tablet film coating contains hypromellose, iron oxide yellow, lactose monohydrate, titanium dioxide, and triacetin. The coated tablets are polished with carnauba wax.
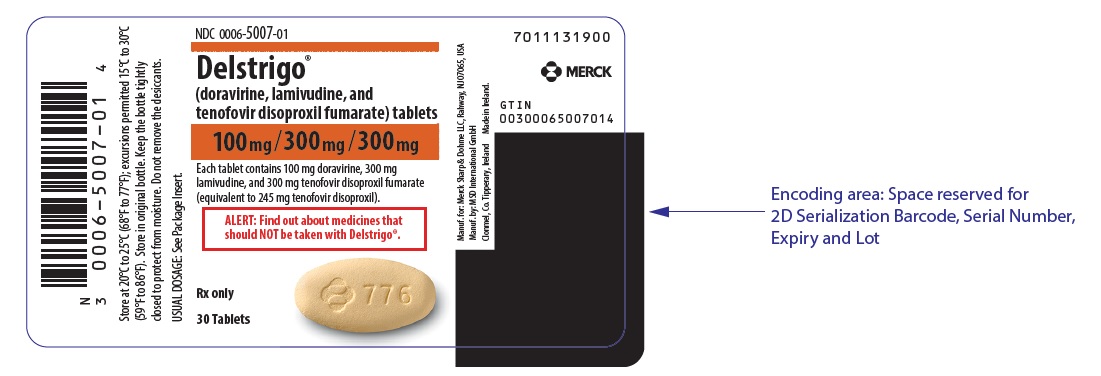
Label
PRINCIPAL DISPLAY PANEL – 30 TABLET BOTTLE LABEL
- NDC 0006-5007-01
- Delstrigo™
(doravirine, lamivudine, and
tenofovir disoproxil fumarate) tablets - 100mg/300mg/300mg
- Each tablet contains 100 mg doravirine, 300 mg
lamivudine, and 300 mg tenofovir disoproxil fumarate
(equivalent to 245 mg tenofovir disoproxil). - ALERT: Find out about medicines that
should NOT be taken with Delstrigo™. - Rx only
- 30 Tablets

SRC: NLM .
