Cresemba
Generic name: isavuconazonium
Drug class: Azole antifungals
Medically reviewed by A Ras MD.
What is Cresemba?
Cresemba is a prescription medicine used to treat people 18 years of age and older with certain types of fungal infections in the blood or body called “aspergillosis,” and “mucormycosis” (zygomycosis).
It is not known if Cresemba is safe and effective in children under 18 years of age.
Description
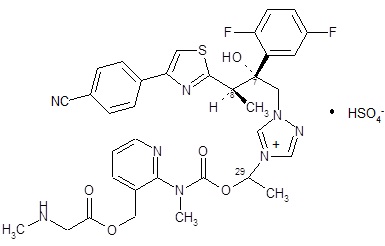
CRESEMBA contains isavuconazonium sulfate, which is the prodrug of isavuconazole, an azole antifungal drug. Isavuconazonium sulfate drug substance is an amorphous, white to yellowish-white powder. The chemical name of isavuconazonium sulfate is glycine, N-methyl-, [2-[[[1-[1-[(2R,3R)-3-[4-(4-cyanophenyl)-2-thiazolyl]-2-(2,5-difluorophenyl)-2-hydroxybutyl]-4H-1,2,4-triazolium-4-yl]ethoxy]carbonyl]methylamino]-3-pyridinyl]methyl ester, sulfate (1:1). The empirical formula is C35H35F2N8O5S·HSO4, the molecular weight is 814.84 and the structural formula is:
CRESEMBA (isavuconazonium sulfate) capsules are available for oral administration. Each CRESEMBA capsule contains 186 mg isavuconazonium sulfate, equivalent to 100 mg isavuconazole. The inactive ingredients include magnesium citrate, microcrystalline cellulose, talc, colloidal silicon dioxide, stearic acid, hypromellose, red iron oxide, titanium dioxide, purified water, gellan gum, potassium acetate, disodium edetate, sodium laurylsulfate, shellac, propylene glycol, strong ammonia solution, potassium hydroxide and black iron oxide.
CRESEMBA (isavuconazonium sulfate) for injection is available for intravenous administration. CRESEMBA for injection is a white to yellow sterile, lyophilized powder containing 372 mg isavuconazonium sulfate, equivalent to 200 mg isavuconazole, per vial. Inactive ingredients included in each vial are 96 mg mannitol and sulfuric acid for pH adjustment.
Who should not take Cresemba?
Do not take Cresemba if you:
- are allergic to Cresemba or any of the ingredients. See the end of this leaflet for a complete list of ingredients in Cresemba.
- are taking any of the following medicines:
- ketoconazole
- high-dose ritonavir
- rifampin
- carbamazepine
- St. John’s wort (herbal supplement)
- long-acting barbiturates
- have a genetic problem that affects the electrical system of the heart (familial short QT syndrome)
Talk to your healthcare provider or pharmacist if you are not sure if you are taking any of these medicines or have any of the conditions listed above.
Do not start taking new medicines without talking to your healthcare provider or pharmacist.
What should I tell my healthcare provider before taking Cresemba?
Before you take Cresemba, tell your healthcare provider about all of your medical conditions, including if you:
- have or ever had an abnormal heart rate or rhythm. Your healthcare provider may order a test to check your heart (ECG) before starting Cresemba.
- have liver problems. Your healthcare provider may do blood tests to make sure you can take Cresemba.
- have ever had an allergic reaction to other antifungal medications such as ketoconazole, fluconazole, itraconazole, voriconazole or posaconazole.
- are pregnant or plan to become pregnant. Cresemba may harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant. Women who can become pregnant should use effective birth control while taking Cresemba and for 28 days after the last Cresemba dose. Talk to your healthcare provider about birth control methods that may be right for you.
- are breastfeeding or plan to breastfeed. Cresemba can pass into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take Cresemba. You should not breastfeed while taking Cresemba.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Cresemba may affect the way other medicines work, and other medicines may affect how Cresemba works and can cause side effects.
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Cresemba?
Take Cresemba exactly as your healthcare provider tells you to take it.
- Do not stop taking Cresemba until your healthcare provider tells you to.
- If you take too much Cresemba, call your healthcare provider.
- Cresemba capsules can be taken with or without food.
- Swallow Cresemba capsules whole.
- Do not chew, crush, dissolve, or open the capsules.
Instructions on opening Cresemba capsules blister packaging:
Cresemba capsules are in child-resistant blister packaging.
Each blister section contains two pockets: one pocket for the Cresemba capsule and one pocket for the desiccant to protect the capsule from moisture (located to the left of the capsule).
- Only open the blister packaging at time of use. Ensure only the Cresemba capsule pocket is opened.
- Do not puncture the pocket containing the desiccant.
- Do not swallow or use the desiccant.
What are the possible side effects of Cresemba?
Cresemba may cause serious side effects, including:
- liver problems. Liver problems can happen in some people taking Cresemba. Some people who also have other serious medical problems may get severe liver problems, which can lead to hepatitis, gallbladder problems, liver failure or death. Your healthcare provider should do blood tests to check your liver before you start and while you are taking Cresemba. Call your healthcare provider right away if you have any of the following symptoms of liver problems:
- infusion reactions. Infusion reactions can happen in people receiving Cresemba intravenously. If an infusion reaction happens, your infusion will be stopped. Symptoms of an infusion reaction may include:
- low blood pressure
- difficulty breathing
- chills
- dizziness
- numbness and tingling
- changes in your sense of touch (hypoesthesia)
- severe allergic and skin reactions.
- drug interactions with cyclosporine, sirolimus, or tacrolimus. If you take Cresemba with cyclosporine, sirolimus, or tacrolimus, your blood levels of cyclosporine, sirolimus, or tacrolimus may increase. Serious side effects can happen in your kidney or brain if you have high levels of cyclosporine, sirolimus, or tacrolimus in your blood. Your healthcare provider should do blood tests to check your levels of cyclosporine, sirolimus, or tacrolimus if you are taking these medicines while taking Cresemba. Tell your healthcare provider right away if you have swelling in your arm or leg or shortness of breath.
- medicine interactions. Taking Cresemba with some other medicines may affect the way other medicines work causing serious side effects. Other medicines may affect the way Cresemba works, causing serious side effects. Tell your healthcare provider about all the medicines you take.
The most common side effects of Cresemba include:
- nausea
- vomiting
- diarrhea
- headache
- changes in the level of a liver enzyme in your blood
- low potassium
- constipation
- shortness of breath
- cough
- swelling of arms or legs
- back pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Cresemba. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Cresemba
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Cresemba for a condition for which it was not prescribed. Do not give Cresemba to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Cresemba. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Cresemba that is written for healthcare professionals.
How should I store Cresemba?
Capsules
- Store Cresemba at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Cresemba in the original package and protect it from moisture.
- Do not remove Cresemba from original packaging.
- Do not put Cresemba in pill boxes or pill organizers.
- Safely throw away medicine that is out of date or no longer needed.
- Keep Cresemba and all medicines out of the reach of children.
Injection
Store Cresemba for injection unreconstituted vials at 2°C to 8°C (36°F to 46°F) in a refrigerator. Cresemba is a single-dose vial of unpreserved sterile lyophile. Following reconstitution of the lyophile with water for injection USP, the reconstituted solution should be used immediately, or stored below 25°C for a maximum of 1 hour prior to preparation of the patient infusion solution. The prepared infusion solution should be kept for not more than 6 hours at room temperature [20°C to 25°C (68°F to 77°F)] or 24 hours at 2°C to 8°C (36°F to 46°F) prior to use. Cresemba for injection vials are for single-dose use only.
What are the ingredients in Cresemba?
Active ingredient: isavuconazonium sulfate.
Inactive ingredients: magnesium citrate, microcrystalline cellulose, talc, colloidal silicon dioxide, stearic acid, hypromellose, red iron oxide, titanium dioxide, purified water, gellan gum, potassium acetate, disodium edetate, sodium laurylsulfate, shellac, propylene glycol, strong ammonia solution, potassium hydroxide, black iron oxide.
Label
PACKAGE/LABEL DISPLAY PANEL
- NDC 0469-0420-01
- CRESEMBA®
(isavuconazonium sulfate) for injection - 372 mg*
- *equivalent to 200 mg isavuconazole
- For Intravenous Infusion Only
Use an in-line filter during infusion
Carefully read the preparation and
administration instructions prior to use - Single Dose Vial
- Discard unused portion
- Rx Only

