Cosopt PF
eneric name: Dorzolamide and Timolol Preservative-Free Eye Drops
Drug class: Ophthalmic glaucoma agents
Medically reviewed by A Ras MD.
What is Cosopt PF?
Cosopt PF is a prescription sterile eye drop solution that contains 2 medicines, dorzolamide hydrochloride (a sulfonamide carbonic anhydrase inhibitor) and timolol maleate (a beta-adrenergic blocker). Cosopt PF is used to lower the pressure in the eye (intraocular pressure) in people with open-angle glaucoma or ocular hypertension, when their eye pressure is too high and beta-adrenergic blocker medicines alone have not adequately lowered the pressure.
It is not known if Cosopt PF is safe and effective in children under 2 years of age.
Description
COSOPT PF (dorzolamide hydrochloride-timolol maleate ophthalmic solution) is the combination of a topical carbonic anhydrase inhibitor and a topical beta-adrenergic receptor blocking agent.
Dorzolamide hydrochloride is described chemically as: (4S-trans)-4-(ethylamino)-5,6-dihydro-6-methyl-4H-thieno[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide monohydrochloride. Dorzolamide hydrochloride is optically active. The specific rotation is:
- [α] 25°C (C=1, water) = ~ -17°.
- 405 nm
Its empirical formula is C10H16N2O4S3•HCl and its structural formula is:
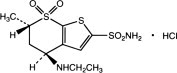
Dorzolamide hydrochloride has a molecular weight of 360.91. It is a white to off-white, crystalline powder, which is soluble in water and slightly soluble in methanol and ethanol.
Timolol maleate is described chemically as: (-)-1-(tert-butylamino)-3-[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol maleate possesses an asymmetric carbon atom in its structure and is provided as the levo-isomer. The optical rotation of timolol maleate is:
- [α] 25°C in 1N HCl (C = 5) = -12.2° (-11.7° to -12.5°).
- 405 nm
Its molecular formula is C13H24N4O3S•C4H4O4 and its structural formula is:
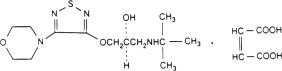
Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol. Timolol maleate is stable at room temperature.
COSOPT PF is supplied as a sterile, clear, colorless to nearly colorless, isotonic, buffered, slightly viscous, aqueous solution. The pH of the solution is approximately 5.65, and the osmolarity is 242-323 mOsM. Each mL of COSOPT PF contains 20 mg dorzolamide (22.26 mg of dorzolamide hydrochloride) and 5 mg timolol (6.83 mg timolol maleate). Inactive ingredients are sodium citrate, hydroxyethyl cellulose, sodium hydroxide, mannitol, and water for injection.
COSOPT PF does not contain a preservative.
Mechanism of Action
COSOPT PF is comprised of two components: dorzolamide hydrochloride and timolol maleate. Each of these two components decreases elevated intraocular pressure, whether or not associated with glaucoma, by reducing aqueous humor secretion. Elevated intraocular pressure is a major risk factor in the pathogenesis of optic nerve damage and glaucomatous visual field loss. The higher the level of intraocular pressure, the greater the likelihood of glaucomatous field loss and optic nerve damage.
Dorzolamide hydrochloride is an inhibitor of human carbonic anhydrase II. Inhibition of carbonic anhydrase in the ciliary processes of the eye decreases aqueous humor secretion, presumably by slowing the formation of bicarbonate ions with subsequent reduction in sodium and fluid transport. Timolol maleate is a beta1 and beta2 (non-selective) adrenergic receptor blocking agent that does not have significant intrinsic sympathomimetic, direct myocardial depressant, or local anesthetic (membrane-stabilizing) activity. The combined effect of these two agents administered as COSOPT PF administered twice daily results in additional intraocular pressure reduction compared to either component administered alone, but the reduction is not as much as when dorzolamide administered three times daily and timolol twice daily are administered concomitantly.
Who should not use Cosopt PF?
Do not use Cosopt PF if you:
- have or have had asthma
- have or have had severe lung problems (chronic obstructive pulmonary disease)
- have heart problems, including slow or irregular heartbeat or heart failure
- are allergic to dorzolamide hydrochloride, timolol maleate, or any of the ingredients in COSOPT PF. See the end of this leaflet for a complete list of ingredients in Cosopt PF.
Talk to your healthcare provider before taking this medicine if you have any of these conditions.
What should I tell my healthcare provider before using Cosopt PF?
Before you use Cosopt PF, tell your doctor if you:
- have problems with muscle weakness (myasthenia gravis)
- have diabetes or problems with low blood sugar (hypoglycemia)
- have thyroid, kidney, or liver problems
- are planning to have surgery
- are allergic to sulfa drugs
- have or have had eye problems, including any surgery on your eye or eyes, or are using any other eye medicines
- have any other medical problems
- are pregnant or plan to become pregnant. It is not known if Cosopt PF will harm your unborn baby. If you become pregnant while using Cosopt PF talk to your doctor right away.
- are breastfeeding or plan to breastfeed. It is not known whether dorzolamide passes into your breast milk however, timolol has been detected in breast milk. Talk to your doctor about the best way to feed your baby if you use Cosopt PF.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Cosopt PF and other medicines may affect each other causing side effects. Cosopt PF may affect the way other medicines work, and other medicines may affect how Cosopt PF works.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use Cosopt PF?
Read the Instructions for Use that come with Cosopt PF for additional instructions about the right way to use Cosopt PF.
- Use Cosopt PF exactly as your doctor tells you.
- Use 1 drop of Cosopt PF in your eye (or eyes) in the morning and 1 drop in the evening.
- If you use other medicines in your eye, wait at least 5 minutes between using Cosopt PF and your other eye medicines.
- Use your Cosopt PF right away after opening. Each Cosopt PF single-use container is sterile and is to be used 1 time then thrown away.
- Do not save any Cosopt PF that may be left over after you use a single-use container. Using Cosopt PF that is not sterile may cause other eye problems.
What are the possible side effects of Cosopt PF?
Cosopt PF may cause serious side effects including:
- severe breathing problems. These breathing problems can happen in people who have asthma, chronic obstructive pulmonary disease, or heart failure and can cause death. Tell your doctor right away if you have breathing problems while taking Cosopt PF.
- heart failure. This can happen in people who already have heart failure and in people who have never had heart failure before.
Tell your doctor right away if you get any of these symptoms of heart failure while taking Cosopt PF:- shortness of breath
- irregular heartbeat (palpitations)
- swelling of your ankles or feet
- sudden weight gain
- severe allergic reactions. These allergic reactions can happen the first time you use Cosopt PF or after you have been using Cosopt PF for a while and may cause death. Stop taking Cosopt PF and call your doctor right away or get emergency help if you get any of these symptoms of an allergic reaction:
- swelling of your face, lips, mouth, or tongue
- trouble breathing
- wheezing
- severe itching
- skin rash, redness, or swelling
- dizziness or fainting
- fast heartbeat or pounding in your chest (tachycardia)
- sweating
- worsening muscle weakness. Cosopt PF can cause muscle weakness to get worse in people who already have problems with muscle weakness (myasthenia gravis).
- kidney problems. Your doctor may do tests to check your kidney function while you use Cosopt PF.
- swelling of your eye (cornea)
The most common side effects of Cosopt PF include:
- a bitter, sour, or unusual taste in your mouth after using Cosopt PF
- burning, stinging, redness, or itching of the eye
- blurred vision
- painful, red, watery eyes with increased sensitivity (superficial punctate keratitis)
Tell your doctor if you have any new eye problems while using Cosopt PF including:
- an eye injury
- an eye infection
- a sudden loss of vision
- eye surgery
- swelling and redness of and around your eye (conjunctivitis)
- problems with your eyelids
Tell your doctor if you have any other side effects that bother you.
These are not all the possible side effects of Cosopt PF. For more information, ask your doctor or pharmacist.
Call your doctor about medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What should I do in case of an overdose?
If you swallow the contents of the container, contact your doctor immediately. Among other effects, you may feel light-headed, have difficulty breathing, or feel your heart rate has slowed.
General information about the safe and effective use of Cosopt PF
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Cosopt PF for a condition for which it was not prescribed. Do not give Cosopt PF to other people, even if they have the same symptoms you have. It may harm them. This Patient Information leaflet summarizes the most important information about Cosopt PF. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Cosopt PF that is written for health professionals.
How should I store Cosopt PF?
- Store Cosopt PF at room temperature between 68°F to 77°F (20°C to 25°C). Do not freeze.
- Keep the Cosopt PF single-use containers in their original foil pouch to protect from light.
- Write down the date you open the foil pouch in the space provided on the pouch.
- Throw away all unused Cosopt PF single-use containers 15 days after first opening the pouch.
Keep Cosopt PF and all medicines out of the reach of children.
What are the ingredients in Cosopt PF?
Active ingredients: dorzolamide hydrochloride and timolol maleate
Inactive ingredients: sodium citrate, hydroxyethyl cellulose, sodium hydroxide, mannitol, and water for injection.
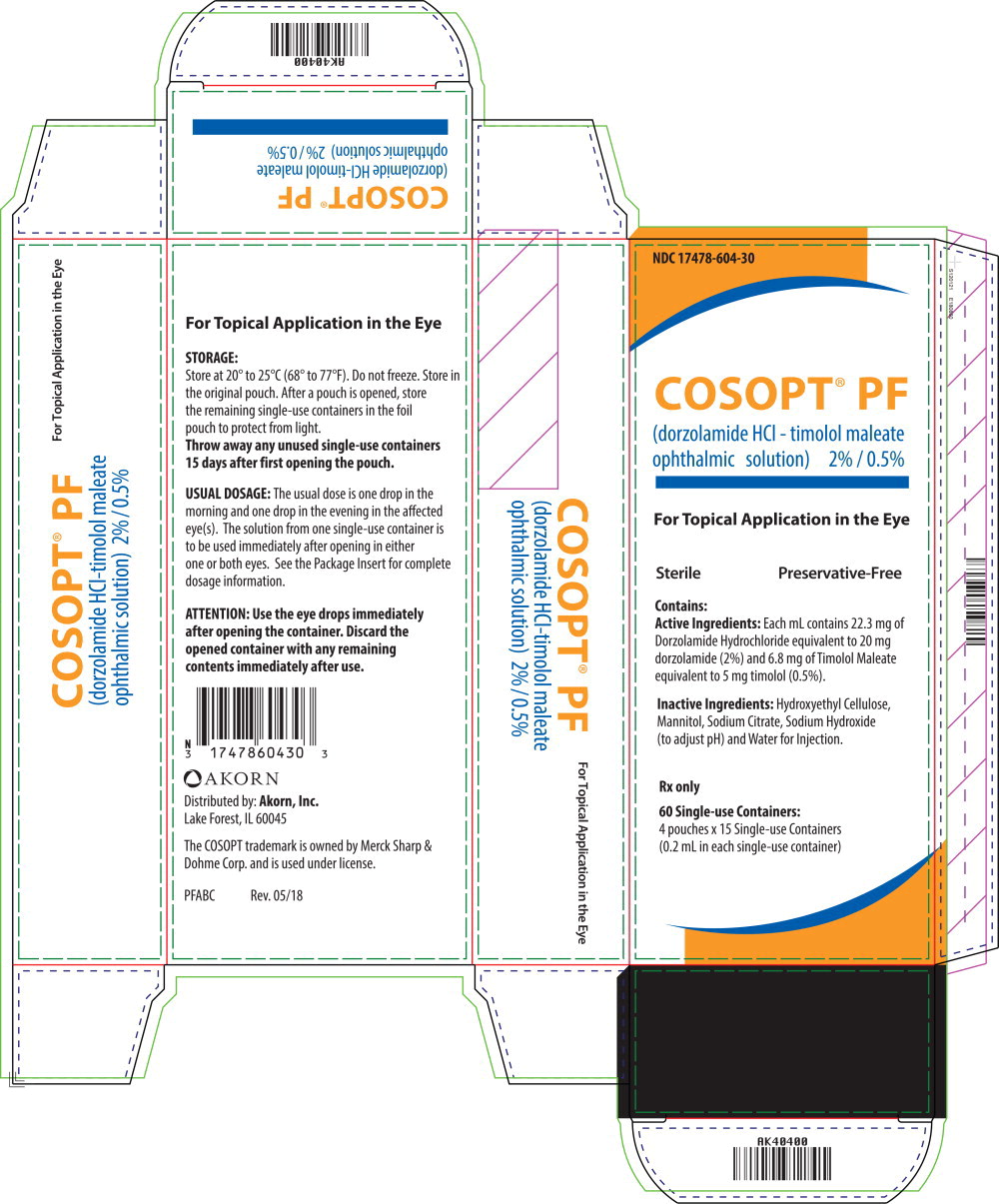
Label
Principal Display Panel Text for Carton Label:
- NDC 17478-604-30
- COSOPT® PF
- (dorzolamide HCl – timolol maleate
- ophthalmic solution) 2% / 0.5%
- For Topical Application in the Eye
- Sterile Preservative-Free
SRC: NLM .
