Copiktra
Generic name: duvelisib
Drug class: PI3K inhibitors
Medically reviewed by A Ras MD.
What is Copiktra?
Copiktra is a prescription medicine used to treat adults with Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) who have received at least 2 prior therapies and they did not work or are no longer working, Follicular Lymphoma (FL) who have received at least 2 prior therapies and they did not work or are no longer working.
It is not known if Copiktra is safe and effective in children less than 18 years of age.
Description
COPIKTRA (duvelisib) is a kinase inhibitor.
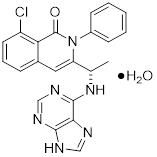
Duvelisib is a white-to-off-white crystalline solid with the empirical formula C22H17ClN6O•H2O and a molecular weight of 434.88 g/mol. Hydration can vary with relative humidity. Duvelisib contains a single chiral center as (S) enantiomer. Duvelisib is soluble in ethanol and practically insoluble in water. Duvelisib is described chemically as a hydrate of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one and has the following chemical structure:
COPIKTRA capsules are for oral administration and are supplied as white to off-white opaque and Swedish orange opaque capsules (25 mg, on anhydrous basis) or pink opaque capsules (15 mg, on anhydrous basis), and contain the following inactive ingredients: colloidal silicon dioxide, crospovidone, magnesium stearate, and microcrystalline cellulose. Capsule shells contain gelatin, titanium dioxide, black ink, and red iron oxide.
Mechanism of Action
Duvelisib is an inhibitor of PI3K with inhibitory activity predominantly against PI3K-δ and PI3K-γ isoforms expressed in normal and malignant B-cells. Duvelisib induced growth inhibition and reduced viability in cell lines derived from malignant B-cells and in primary CLL tumor cells. Duvelisib inhibits several key cell-signaling pathways, including B-cell receptor signaling and CXCR12-mediated chemotaxis of malignant B-cells. Additionally, duvelisib inhibits CXCL12-induced T cell migration and M-CSF and IL-4 driven M2 polarization of macrophages.
What is the most important information I should know about Copiktra?
Copiktra can cause serious side effects, including:
- Infections. Infections are common during Copiktra treatment, and can be serious and can lead to death. Tell your healthcare provider right away if you have a fever, chills, or other signs of an infection during treatment with Copiktra.
- Diarrhea or inflammation of your intestine. Diarrhea or inflammation of your intestine (colitis) is common during Copiktra treatment, and can be serious and can lead to death. Your healthcare provider may prescribe an anti-diarrhea medicine for your diarrhea. Tell your healthcare provider right away if you have any new or worsening diarrhea, stool with mucus or blood, or if you have severe stomach-area (abdominal) pain. Your healthcare provider should prescribe medicine to help your diarrhea and check you at least weekly. If your diarrhea is severe or anti-diarrhea medicines did not work, you may need treatment with a steroid medicine.
- Skin reactions. Rashes are common with Copiktra treatment. Copiktra can cause rashes and other skin reactions that can be serious and can lead to death. Tell your healthcare provider right away if you get a new or worsening skin rash, or other skin reactions during treatment with Copiktra, including:
- painful sores or ulcers on your skin, lips, or in your mouth
- severe rash with blisters or peeling skin
- rash with itching
- rash with fever
Your healthcare provider may need to prescribe medicines, including a steroid medicine, to help treat your skin rash or other skin reactions.
- Inflammation of the lungs. Copiktra can cause inflammation of your lungs which can be serious and can lead to death. Tell your healthcare provider right away if you get new or worsening cough or difficulty breathing. Your healthcare provider may do tests to check your lungs if you have breathing problems during treatment with Copiktra. Your healthcare provider may treat you with a steroid medicine if you develop inflammation of the lungs that is not due to an infection.
If you have any of the above serious side effects during treatment with Copiktra, your healthcare provider may stop your treatment for a period of time, change your dose of Copiktra, or completely stop your treatment with Copiktra. See “What are the possible side effects of Copiktra?” for more information about side effects.
What should I tell my healthcare provider before taking Copiktra?
Before taking Copiktra, tell your healthcare provider about all of your medical conditions, including if you:
- have intestinal problems
- have lung or breathing problems
- have an infection
- are pregnant or plan to become pregnant. Copiktra can harm your unborn baby.
- Your healthcare provider should do a pregnancy test to see if you are pregnant before you start treatment with Copiktra.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with Copiktra and for at least 1 month after the last dose of Copiktra. Talk to your healthcare provider about birth control methods that may be right for you. Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with Copiktra.
- Males with female partners who are able to become pregnant should use effective birth control (contraception) during treatment with Copiktra and for at least 1 month after the last dose of Copiktra.
- are breastfeeding or plan to breastfeed. It is not known if Copiktra passes into breast milk. Do not breastfeed during treatment and for at least 1 month after the last dose of Copiktra.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Copiktra and certain other medicines may affect each other.
How should I take Copiktra?
- Take Copiktra exactly the way your healthcare provider tells you.
- Your healthcare provider may change your dose of Copiktra or tell you to stop taking Copiktra. Do not change your dose or stop taking Copiktra without talking to your healthcare provider first.
- Swallow Copiktra capsules whole.
- Do not open, break, or chew Copiktra capsules.
- You may take Copiktra with or without food.
- Do not miss a dose of Copiktra. If you miss a dose of Copiktra by less than 6 hours, take the missed dose right away, and then take the next dose at your usual time. If you miss a dose by more than 6 hours, wait and take the next dose at your usual time.
- If you take too much Copiktra, call your healthcare provider right away or go to the nearest hospital emergency room.
What are the possible side effects of Copiktra?
Copiktra may cause serious side effects, including:
- See “What is the most important information I should know about Copiktra?”
- Elevated liver enzymes. Copiktra may cause abnormalities in liver blood tests. Your healthcare provider should do blood tests during your treatment with Copiktra to check for liver problems. Tell your healthcare provider right away if you get any symptoms of liver problems, including yellowing of your skin or the white part of your eyes (jaundice), pain in the abdominal region, bruising or bleeding more easily than normal.
- Low white blood cell count (neutropenia). Neutropenia is common with Copiktra treatment and can sometimes be serious. Your healthcare provider should check your blood counts regularly during treatment with Copiktra. Tell your healthcare provider right away if you have a fever or any signs of infection during treatment with Copiktra.
Common side effects of Copiktra include:
- tiredness
- fever
- cough
- nausea
- upper respiratory infection
- bone and muscle pain
- low red blood cell count
These are not all the possible side effects of Copiktra.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Copiktra
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Copiktra for a condition for which it was not prescribed. Do not give Copiktra to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Copiktra that is written for health professionals.
How should I store Copiktra?
- Store Copiktra at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep Copiktra in its original container until you are ready to take your dose.
Keep Copiktra and all medicines out of the reach of children.
What are the ingredients in Copiktra?
Active ingredient: duvelisib
Inactive ingredients: Colloidal silicon dioxide, crospovidone, magnesium stearate, and microcrystalline cellulose.
Capsule shells contain gelatin, titanium dioxide, black ink, and red iron oxide.
Label
PRINCIPAL DISPLAY PANEL – NDC: 73116-215-28 – 15 MG CAPSULE 14 DAYS PACK BLISTER PACK LABEL