Cabometyx
Generic name: cabozantinib
Drug classes: Multikinase inhibitors, VEGF/VEGFR inhibitors
Medically reviewed by A Ras MD.
What is Cabometyx?
Cabometyx is a prescription medicine used to treat people with kidney cancer (renal cell carcinoma). Cabometyx may be used alone to treat people with renal cell carcinoma (RCC) that has spread (advanced RCC), in combination with nivolumab when your cancer has spread (advanced RCC), and you have not already had treatment for your advanced RCC.
It is also used to treat people with liver cancer (hepatocellular carcinoma) who have been previously treated with the medicine sorafenib, adults and children 12 years of age and older who have a type of thyroid cancer called differentiated thyroid cancer (DTC) that has spread (locally advanced or metastatic), and, has progressed after treatment with a VEGFR-targeted treatment, and your DTC can no longer be treated with radioactive iodine, or you are not able to receive radioactive iodine treatment.
It is not known if Cabometyx is safe and effective in children younger than 12 years of age.
Description
CABOMETYX is the (S)-malate salt of cabozantinib, a kinase inhibitor. Cabozantinib (S)-malate is described chemically as N-(4-(6,7-dimethoxyquinolin-4-yloxy)phenyl)-N’-(4-fluorophenyl)cyclopropane-1,1-dicarboxamide, (2S)-hydroxybutanedioate. The molecular formula is C28H24FN3O5•C4H6O5 and the molecular weight is 635.6 Daltons as malate salt. The chemical structure of cabozantinib (S)-malate salt is:
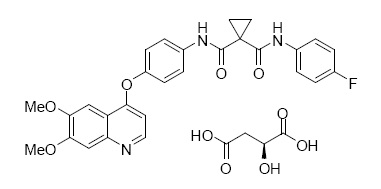
Cabozantinib (S)-malate salt is a white to off-white solid that is practically insoluble in aqueous media.
CABOMETYX (cabozantinib) tablets for oral use are supplied as film-coated tablets containing 20 mg, 40 mg, or 60 mg of cabozantinib, which is equivalent to 25 mg, 51 mg, or 76 mg of cabozantinib (S)-malate, respectively. CABOMETYX also contains the following inactive ingredients: microcrystalline cellulose, lactose anhydrous, hydroxypropyl cellulose, croscarmellose sodium, colloidal silicon dioxide, and magnesium stearate.
The film coating contains hypromellose, titanium dioxide, triacetin, and iron oxide yellow.
Mechanism of Action
In vitro biochemical and/or cellular assays have shown that cabozantinib inhibits the tyrosine kinase activity of MET, VEGFR-1, -2 and -3, AXL, RET, ROS1, TYRO3, MER, KIT, TRKB, FLT-3, and TIE-2. These receptor tyrosine kinases are involved in both normal cellular function and pathologic processes such as oncogenesis, metastasis, tumor angiogenesis, drug resistance, and maintenance of the tumor microenvironment.
What is the most important information I should know about Cabometyx?
If your healthcare provider prescribes Cabometyx in combination with nivolumab, also read the Medication Guide that comes with nivolumab.
What should I tell my healthcare provider before taking Cabometyx?
Before you take Cabometyx, tell your healthcare provider about all of your medical conditions, including if you:
- have had a liver problem other than liver cancer
- have a recent history of bleeding, including coughing up or vomiting blood, or black tarry stools
- have an open or healing wound
- have high blood pressure
- have low calcium in your blood (hypocalcemia)
- plan to have any surgery, dental procedure, or have had a recent surgery. You should stop taking Cabometyx at least 3 weeks before planned surgery. See “What are the possible side effects of Cabometyx?”
- are pregnant, or plan to become pregnant. Cabometyx can harm your unborn baby.
- If you are able to become pregnant, your healthcare provider will check your pregnancy status before you start treatment with Cabometyx.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment and for 4 months after your final dose of Cabometyx.
- Talk to your healthcare provider about birth control methods that may be right for you.
- If you become pregnant or think you are pregnant, tell your healthcare provider right away.
- are breastfeeding or plan to breastfeed. It is not known if Cabometyx passes into your breast milk. Do not breastfeed during treatment and for 4 months after your final dose of Cabometyx.
Tell your healthcare provider about all the medicines you take, including prescription or over-the- counter medicines, vitamins, and herbal supplements. Cabometyx and certain other medicines may affect each other causing side effects.
How should I take Cabometyx?
- Take Cabometyx exactly as your healthcare provider tells you to take it.
- Do not take Cabometyx with food. Take Cabometyx at least 1 hour before or at least 2 hours after eating.
- Swallow Cabometyx tablets whole with a full glass (at least 8 ounces) of water.
- Do not crush Cabometyx tablets.
- If you miss a dose and your next dose is in less than 12 hours, take your next dose at the normal time. Do not make up the missed dose.
What should I avoid while taking Cabometyx?
Avoid drinking grapefruit juice, eating grapefruit or taking supplements that contain grapefruit or St. John’s wort during treatment with Cabometyx.
What are the possible side effects of Cabometyx?
Cabometyx may cause serious side effects, including:
- bleeding (hemorrhage). Cabometyx can cause severe bleeding that may lead to death. Tell your healthcare provider right away if you get any signs of bleeding during treatment with Cabometyx, including:
- coughing up blood or blood clots
- vomiting blood or if your vomit looks like coffee-grounds
- red or black (looks like tar) stools
- menstrual bleeding that is heavier than normal
- any unusual or heavy bleeding
- a tear in your stomach or intestinal wall (perforation) or an abnormal connection between 2 parts of your body (fistula). Tell your healthcare provider right away if you get tenderness or pain in your stomach-area (abdomen) that is severe or that does not go away.
- blood clots, stroke, heart attack, and chest pain. Get emergency help right away if you get:
- swelling or pain in your arms or legs
- shortness of breath
- feel lightheaded or faint
- sweating more than usual
- numbness or weakness of your face, arm or leg, especially on one side of your body
- sudden confusion, trouble speaking or understanding
- sudden trouble seeing in one or both eyes
- sudden trouble walking
- dizziness, loss of balance or coordination
- a sudden severe headache
- high blood pressure (hypertension). Hypertension is common with Cabometyx and sometimes can be severe. Your healthcare provider will check your blood pressure before starting Cabometyx and during treatment with Cabometyx. If needed, your healthcare provider may prescribe medicine to treat your high blood pressure. Tell your healthcare provider if you develop severe headaches, nose bleeds, tiredness or confusion, vision changes, chest pain, trouble breathing, irregular heartbeat, or blood in your urine.
- diarrhea. Diarrhea is common with Cabometyx and can be severe. If needed, your healthcare provider may prescribe medicine to treat your diarrhea. Tell your healthcare provider right away, if you have frequent loose, watery bowel movements.
- a skin problem called hand-foot skin reaction. Hand-foot skin reactions are common and can be severe. Tell your healthcare provider right away if you have rashes, redness, pain, swelling, or blisters on the palms of your hands or soles of your feet.
- liver problems. Liver problems may happen during treatment with Cabometyx. When Cabometyx is taken in combination with nivolumab, severe changes in liver function tests may happen more often than if you take Cabometyx alone. Your healthcare provider will do blood tests to check your liver function before and during treatment with Cabometyx. Tell your healthcare provider right away if you develop symptoms of liver problems including: yellowing of your skin or the whites of your eyes, severe nausea or vomiting, pain on the right side of your stomach-area (abdomen), dark urine, bleeding or bruising more easily than normal.
- adrenal gland problems. Your healthcare provider will monitor you for this problem. Your healthcare provider may prescribe hormone replacement therapy or corticosteroid medicines if needed. Tell your healthcare provider right away if you develop any of the following signs or symptoms: extreme tiredness, dizziness or fainting, weakness, nausea, or vomiting.
- protein in your urine and possible kidney problems. Symptoms may include swelling in your hands, arms, legs, or feet. Your healthcare provider will check you for this problem during treatment with Cabometyx.
- severe jaw bone problems (osteonecrosis). Your healthcare provider should examine your mouth before you start and during treatment with Cabometyx. Tell your dentist that you are taking Cabometyx. It is important for you to practice good mouth care during treatment with Cabometyx. Tell your healthcare provider right away if you develop any symptoms of jaw 47 problems, including: jaw pain, toothache, or sores on your gums.
- wound healing problems. Wound healing problems have happened in some people who take Cabometyx. Tell your healthcare provider if you plan to have any surgery before or during treatment with Cabometyx.
- You should stop taking Cabometyx at least 3 weeks before planned surgery.
- Your healthcare provider should tell you when you may start taking Cabometyx again after surgery.
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS). A condition called reversible posterior leukoencephalopathy syndrome can happen during treatment with Cabometyx. Tell your healthcare provider right away if you have headaches, seizures, confusion, changes in vision, or problems thinking.
- change in thyroid function. Cabometyx can cause changes in your thyroid function, including changes to thyroid hormone levels in your blood. Your healthcare provider will do blood tests to check your thyroid function before and during treatment with Cabometyx.
- decreased calcium level in your blood (hypocalcemia). Cabometyx can cause you to have a decreased amount of calcium in your blood. Your healthcare provider will do blood tests to check you for this problem and give you calcium if needed. Tell your healthcare provider right away if you get any of the following signs or symptoms:
- muscle stiffness or muscle spasms
- sudden weight gain
- numbness or tingling in your fingers, toes, or around your mouth
- swelling of your arms, hands, legs, and ankles
- seizures
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Cabometyx if you have certain side effects.
The most common side effects of Cabometyx include:
- tiredness
- decreased appetite
- nausea and vomiting
- weight loss
- constipation
The most common side effects of Cabometyx when used in combination with nivolumab include:
- tiredness
- mouth sores
- rash
- low thyroid hormone levels (hypothyroidism)
- pain in muscles, bones, and joints
- decreased appetite
- nausea
- changes in the way things taste
- stomach-area (abdominal) pain
- cough
- upper respiratory tract infection
Cabometyx may cause fertility problems in females and males, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility. These are not all of the possible side effects of Cabometyx. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Cabometyx
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Cabometyx for a condition for which it was not prescribed. Do not give Cabometyx to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Cabometyx that is written for health professionals.
How should I store Cabometyx?
- Store Cabometyx at room temperature 68°F to 77°F (20°C to 25°C).
Keep Cabometyx and all medicines out of the reach of children.
What are the ingredients in Cabometyx?
Active ingredient: cabozantinib
Inactive ingredients: microcrystalline cellulose, lactose anhydrous, hydroxypropyl cellulose, croscarmellose sodium, colloidal silicon dioxide, and magnesium stearate. The film coating contains hypromellose, titanium dioxide, triacetin, and iron oxide yellow.
Label
Package Label – Bottle – 30 Tablets – 60mg CABOMETYX
- NDC 42388-023-26
- Cabometyx®
(cabozantinib) tablets60 mg* - Rx Only
30 tablets
Exelixis® - *Each tablet contains 60 mg of
cabozantinib (equivalent to 76 mg of
cabozantinib (S)-malate). - Take once each day on an empty
stomach. Cabometyx should not
be taken with food. Take
Cabometyx at least 1 hour before
or at least 2 hours after eating.
Swallow Cabometyx tablets whole.
Do not crush Cabometyx tablets. - Store Cabometyx at 20°C to 25°C
(68°F to 77°F); excursions are
permitted from 15°C to 30°C
(59°F to 86°F) [see USP Controlled
Room Temperature]. - Keep out of the reach of children.
- Manufactured for Exelixis, Inc.
Alameda, CA 94502
Made in Canada

SRC: NLM .