Cabenuva
Generic name: cabotegravir and rilpivirine
Dosage form: intramuscular suspension, extended release (200 mg-300 mg/mL)
Drug class: Antiviral combinations
What is Cabenuva?
Cabenuva is a prescription medicine used without other Human Immunodeficiency Virus-1 (HIV-1) medicines to treat HIV-1 infection in adults to replace their current HIV-1 medicines when their healthcare provider determines that they meet certain requirements.
HIV-1 is the virus that causes Acquired Immune Deficiency Syndrome (AIDS).
Cabenuva contains 2 different medicines cabotegravir, rilpivirine. It is not known if Cabenuva is safe and effective in children.
Description
CABENUVA contains cabotegravir extended-release injectable suspension, an HIV INSTI, co-packaged with rilpivirine extended-release injectable suspension, an HIV NNRTI.
Cabotegravir
The chemical name for cabotegravir is (3S,11aR)-N-[(2,4-difluorophenyl)methyl]-6-hydroxy-3-methyl-5,7-dioxo-2,3,5,7,11,11a-hexahydro[1,3]oxazolo[3,2-a]pyrido[1,2-d]pyrazine-8-carboxamide. The empirical formula is C19H17F2N3O5 and the molecular weight is 405.35 g/mol. It has the following structural formula:
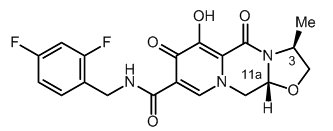
Cabotegravir extended-release injectable suspension is a white to light pink free-flowing suspension for intramuscular injection. Each sterile single-dose vial contains 2 mL or 3 mL of the following: cabotegravir 200 mg/mL and the following inactive ingredients: mannitol (35 mg/mL), polyethylene glycol (PEG) 3350 (20 mg/mL), polysorbate 20 (20 mg/mL), and Water for Injection.
Rilpivirine
The chemical name for rilpivirine is 4-[[4-[[4-[(E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile. Its molecular formula is C22H18N6 and its molecular weight is 366.42. Rilpivirine has the following structural formula:
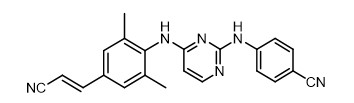
Rilpivirine extended-release injectable suspension is a white to off-white suspension for intramuscular injection. Each sterile single-dose vial contains 2 mL or 3 mL of the following: rilpivirine 300 mg/mL and the following inactive ingredients: citric acid monohydrate (1 mg/mL), poloxamer 338 (50 mg/mL), Water for Injection, glucose monohydrate to ensure isotonicity, sodium dihydrogen phosphate monohydrate, and sodium hydroxide to adjust pH.
The vial stoppers are not made with natural rubber latex.
Who should not use Cabenuva?
Do not receive Cabenuva if you:
- have ever had an allergic reaction to cabotegravir or rilpivirine.
- are taking any of the following medicines:
- carbamazepine
- oxcarbazepine
- phenobarbital
- phenytoin
- rifabutin
- rifampin
- rifapentine
- dexamethasone (more than a single dose treatment)
- St John’s wort (Hypericum perforatum)
What should I tell my healthcare provider before using Cabenuva?
Before you receive Cabenuva, tell your healthcare provider about all your medical conditions, including if you:
- have ever had a skin rash or an allergic reaction to medicines that contain cabotegravir or rilpivirine.
- have or have had liver problems, including hepatitis B or C infection.
- have ever had mental health problems.
- are pregnant or plan to become pregnant. It is not known if Cabenuva will harm your unborn baby. Cabenuva can remain in your body for up to 12 months or longer after the last injection.
Pregnancy Registry. There is a pregnancy registry for women who take Cabenuva during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry. - are breastfeeding or plan to breastfeed. Do not breastfeed if you take Cabenuva.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV-1 to your baby.
- It is not known if Cabenuva can pass to your baby in your breast milk. Talk with your healthcare provider about the best way to feed your baby during treatment with Cabenuva.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Some medicines interact with Cabenuva. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine. You can ask your healthcare provider or pharmacist for a list of medicines that interact with Cabenuva.
Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take Cabenuva with other medicines.
How should I use Cabenuva?
- Your healthcare provider will inject Cabenuva into the muscle of each side of your buttocks.
- You will receive Cabenuva as 2 injections (cabotegravir and rilpivirine), one time every month.
- Before receiving your first injection doses of Cabenuva, your healthcare provider will have you take 1 Vocabria (cabotegravir) tablet and 1 Edurant (rilpivirine) tablet one time a day for one month (at least 28 days). This will allow your healthcare provider to assess how well you tolerate these medicines.
- Cabenuva is a long-acting medicine and may stay in your system for 12 months or longer after your last injection.
- Stay under the care of a healthcare provider during treatment with Cabenuva. It is important that you attend your planned appointments to receive your injection doses of Cabenuva.
- If you miss or plan to miss a scheduled monthly injection of Cabenuva by more than 7 days, call your healthcare provider right away to discuss your treatment options.
- If you stop treatment with Cabenuva you will need to take other medicines to treat your HIV-1 infection and reduce the risk of developing viral resistance. Call your healthcare provider right away to discuss your treatment options.
What are the possible side effects of Cabenuva?
Cabenuva may cause serious side effects including:
- Allergic reactions. Call your healthcare provider right away if you develop a rash with Cabenuva. Stop receiving Cabenuva and get medical help right away if you develop a rash with any of the following signs or symptoms:
- fever
- generally ill feeling
- tiredness
- muscle or joint aches
- trouble breathing
- blisters or sores in mouth
- blisters
- redness or swelling of the eyes
- swelling of the mouth, face, lips, or tongue
- Post-injection reactions. Post-injection reaction symptoms have happened within minutes in some people after receiving their rilpivirine injection. Most symptoms resolved within a few minutes after the injection. Symptoms of post-injection reactions may include:
- trouble breathing
- stomach cramps
- sweating
- numbness of your mouth
- feeling anxious
- feeling warm
- feeling lightheaded or feeling like you are going to pass out (faint)
- blood pressure changes
- Liver problems. People with a history of hepatitis B or C virus or people who have certain liver function test changes may have an increased risk of developing new or worsening changes in certain liver tests during treatment with Cabenuva. Liver problems have also happened in people without history of liver problems or other risk factors. Your healthcare provider may do blood tests to check your liver function.
Call your healthcare provider right away if you develop any of the following signs or symptoms of liver problems: - Depression or mood changes.Call your healthcare provider or get emergency medical help right away if you have any of the following symptoms:
- feeling sad or hopeless
- feeling anxious or restless
- have thoughts of hurting yourself (suicide) or have tried to hurt yourself
The most common side effects of Cabenuva include:
- Pain, tenderness, hardened mass or lump, swelling, redness, itching, bruising, and warmth at the injection site
- fever
- tiredness
- headache
- muscle or bone pain
- nausea
- sleep problems
- dizziness
- rash
These are not all the possible side effects of Cabenuva. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
General information about the safe and effective use of Cabenuva
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your healthcare provider or pharmacist for information about Cabenuva that is written for health professionals.
What are the ingredients in Cabenuva?
Cabotegravir extended-release injectable suspension:
Active ingredient: cabotegravir
Inactive ingredients: mannitol, polyethylene glycol (PEG) 3350, polysorbate 20, and Water for Injection.
Rilpivirine extended-release injectable suspension:
Active ingredient: rilpivirine
Inactive ingredients: citric acid monohydrate, poloxamer 338, Water for Injection, glucose monohydrate to ensure isotonicity, sodium dihydrogen phosphate monohydrate, and sodium hydroxide to adjust pH.
SRC: NLM .
