Briviact
Generic name: brivaracetam (oral/injection)
Drug class: Pyrrolidine anticonvulsants
Medically reviewed by A Ras MD.
What is Briviact?
Briviact is a prescription medicine used to treat partial-onset seizures in people 4 years of age and older.
It is not known if Briviact injection is safe for use in children. Children 4 years of age and older should only take Briviact by mouth.
Briviact injection is only for use in people 16 years of age and older and may be given in the vein (intravenously) when Briviact is not able to be taken by mouth. It is not known if Briviact is safe and effective in children younger than 4 years of age.
Description
The chemical name of BRIVIACT (brivaracetam) is (2S)-2-[(4R)-2-oxo-4-propyltetrahydro-1H-pyrrol-1-yl] butanamide. Its molecular formula is C11H20N2O2 and its molecular weight is 212.29. The chemical structure is:
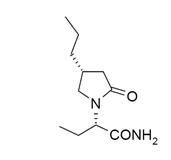
Brivaracetam is a white to off-white crystalline powder. It is very soluble in water, buffer (pH 1.2, 4.5, and 7.4), ethanol, methanol, and glacial acetic acid. It is freely soluble in acetonitrile and acetone and soluble in toluene. It is very slightly soluble in n-hexane.
Tablets
BRIVIACT tablets are for oral administration and contain the following inactive ingredients: croscarmellose sodium, lactose monohydrate, betadex (β-cyclodextrin), anhydrous lactose, magnesium stearate, and film coating agents specified below:
10 mg tablets: polyvinyl alcohol, talc, polyethylene glycol 3350, titanium dioxide
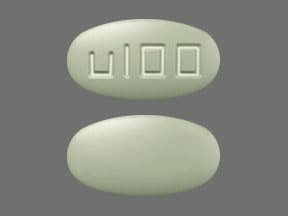
25 mg and 100 mg tablets: polyvinyl alcohol, talc, polyethylene glycol 3350, titanium dioxide, yellow iron oxide, black iron oxide
50 mg tablets: polyvinyl alcohol, talc, polyethylene glycol 3350, titanium dioxide, yellow iron oxide, red iron oxide
75 mg tablets: polyvinyl alcohol, talc, polyethylene glycol 3350, titanium dioxide, yellow iron oxide, red iron oxide, black iron oxide
Oral Solution
BRIVIACT oral solution contains 10 mg of brivaracetam per mL. The inactive ingredients are sodium citrate, anhydrous citric acid, methylparaben, sodium carboxymethylcellulose, sucralose, sorbitol solution, glycerin, raspberry flavor, and purified water.
Injection
BRIVIACT injection is a clear, colorless liquid provided as a sterile, preservative-free solution. BRIVIACT injection contains 10 mg brivaracetam per mL for intravenous administration. One vial contains 50 mg of brivaracetam drug substance. It contains the following inactive ingredients: sodium acetate, trihydrate (1.64 mg/mL), glacial acetic acid (for pH adjustment to 5.5), sodium chloride (9.00 mg/mL), and water for injection.
Mechanism of Action
The precise mechanism by which BRIVIACT exerts its anticonvulsant activity is not known. Brivaracetam displays a high and selective affinity for synaptic vesicle protein 2A (SV2A) in the brain, which may contribute to the anticonvulsant effect.
What is the most important information I should know about Briviact?
Briviact is a federally controlled substance (CV) because it can be abused or lead to dependence. Keep Briviact in a safe place to prevent misuse and abuse. Selling or giving away Briviact may harm others and is against the law.
Like other antiepileptic drugs, Briviact may cause suicidal thoughts or actions in a very small number of people, about 1 in 500 people taking it.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- new or worse depression
- feeling agitated or restless
- trouble sleeping (insomnia)
- acting aggressive, feeling angry, or being violent
- an extreme increase in activity and talking (mania)
- attempts to commit suicide
- new or worse anxiety
- panic attacks
- new or worse irritability
- acting on dangerous impulses
- other unusual changes in behavior or mood
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop Briviact without first talking to a healthcare provider.
- Stopping Briviact suddenly can cause serious problems.
- Stopping a seizure medicine suddenly can cause seizures that will not stop (status epilepticus).
Who should not take Briviact?
Do not take Briviact if you are allergic to brivaracetam or any of the inactive ingredients in Briviact. See the end of this Medication Guide for a complete list of ingredients in Briviact.
What should I tell my healthcare provider before taking Briviact?
Before taking Briviact, tell your healthcare provider about all of your medical conditions, including if you:
- have or had depression, mood problems, or suicidal thoughts or behavior.
- have liver problems.
- have abused or been dependent on prescription medicines, street drugs, or alcohol.
- are pregnant or plan to become pregnant. It is not known if Briviact will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking Briviact. You and your healthcare provider will have to decide if you should take Briviact while you are pregnant. If you become pregnant while taking Briviact, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of Briviact and other antiepileptic medicines during pregnancy.
- are breastfeeding or plan to breastfeed. It is not known if Briviact passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take Briviact.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Briviact may affect the way other medicines work, and other medicines may affect how Briviact works. Do not start a new medicine without first talking with your healthcare provider. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine.
How should I take Briviact?
- Take Briviact exactly as your healthcare provider tells you.
- Your healthcare provider will tell you how much Briviact to take and when to take it.
- Your healthcare provider may change your dose if needed. Do not change your dose without talking to your healthcare provider.
- Take Briviact with or without food.
- Swallow Briviact tablets whole with a liquid. Do not chew or crush Briviact tablets before swallowing.
- If your healthcare provider has prescribed Briviact oral solution, be sure to ask your pharmacist for a medicine dropper or medicine cup to help you measure the correct amount of Briviact oral solution. Do not use a household teaspoon or tablespoon. Ask your pharmacist for instructions on how to use the measuring device the right way.
- Briviact injection can be given to you by intravenous (IV) infusion into your vein, as prescribed by your healthcare provider.
- If you take too much Briviact, call your Poison Control Center or go to the nearest emergency room right away.
What should I avoid while taking Briviact?
Do not drive or operate machinery until you know how Briviact affects you. Briviact may cause drowsiness, tiredness, dizziness, and problems with your balance and coordination.
What are the possible side effects of Briviact?
Briviact may cause serious side effects, including:
- See “What is the most important information I should know about Briviact?”
- Nervous system problems. Drowsiness, tiredness, and dizziness are common with Briviact, but can be severe. See “What should I avoid while taking Briviact?” Briviact can also cause problems with balance and coordination.
- Mental (psychiatric) symptoms. Briviact can cause mood and behavior changes such as aggression, agitation, anger, anxiety, apathy, mood swings, depression, hostility, and irritability. Irritability and anxiety are common with Briviact, and can be severe. People who take Briviact can also get psychotic symptoms such as hallucinations (seeing or hearing things that are really not there), delusions (false or strange thoughts or beliefs), and unusual behavior.
The most common side effects of Briviact in adults include:
Side effects of Briviact in children 4 to less than 16 years of age are similar to those seen in adults.
These are not all the possible side effects of Briviact. For more information, ask your healthcare provider or pharmacist. Tell your healthcare provider about any side effect that bothers you or that does not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Briviact
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Briviact for a condition for which it was not prescribed. Do not give Briviact to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Briviact. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about Briviact that is written for health professionals.
How should I store Briviact?
- Store Briviact at room temperature between 59°F to 86°F (15°C to 30°C).
- Do not freeze Briviact oral solution.
- Safely throw away any opened bottle of Briviact oral solution after 5 months of first opening the bottle, even if there is medicine left in the bottle.
Keep Briviact and all medicines out of the reach of children.
What are the ingredients in Briviact?
Active ingredient: brivaracetam
Tablet inactive ingredients: croscarmellose sodium, lactose monohydrate, betadex (β-cyclodextrin), anhydrous lactose, and magnesium stearate. The tablet film coating contains the inactive ingredients listed below:
- 10 mg tablets: polyvinyl alcohol, talc, polyethylene glycol 3350, titanium dioxide
- 25 mg and 100 mg tablets: polyvinyl alcohol, talc, polyethylene glycol 3350, titanium dioxide, yellow iron oxide, black iron oxide
- 50 mg tablets: polyvinyl alcohol, talc, polyethylene glycol 3350, titanium dioxide, yellow iron oxide, red iron oxide
- 75 mg tablets: polyvinyl alcohol, talc, polyethylene glycol 3350, titanium dioxide, yellow iron oxide, red iron oxide, black iron oxide
Oral solution inactive ingredients: sodium citrate, anhydrous citric acid, methylparaben, sodium carboxymethylcellulose, sucralose, sorbitol solution, glycerin, raspberry flavor, and purified water.
Injection inactive ingredients: sodium acetate (trihydrate), glacial acetic acid, sodium chloride, and water for injection.
Label
PRINCIPAL DISPLAY PANEL – 10 MG TABLET BOTTLE CARTON
- NDC 50474-370-66
Rx only - BRIVIACT®
(brivaracetam)
tablets - CV
- 10 mg
- ATTENTION PHARMACIST:
Dispense accompanying
medication guide to each patient. - 60 tablets
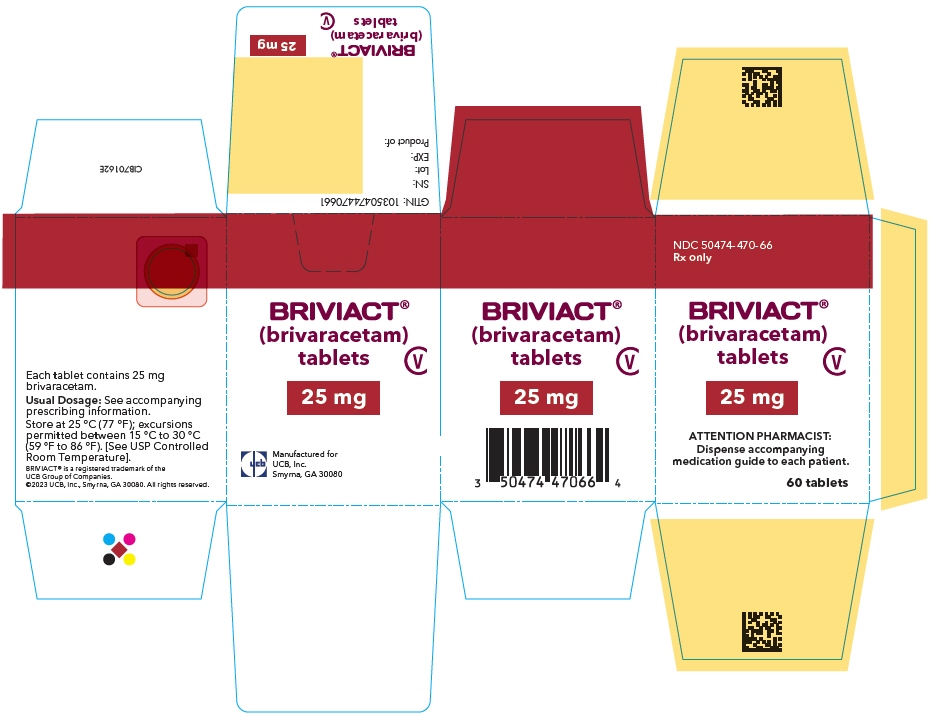
PRINCIPAL DISPLAY PANEL – 25 MG TABLET BOTTLE CARTON
- NDC 50474-470-66
Rx only - BRIVIACT®
(brivaracetam)
tablets - CV
- 25 mg
- ATTENTION PHARMACIST:
Dispense accompanying
medication guide to each patient. - 60 tablets
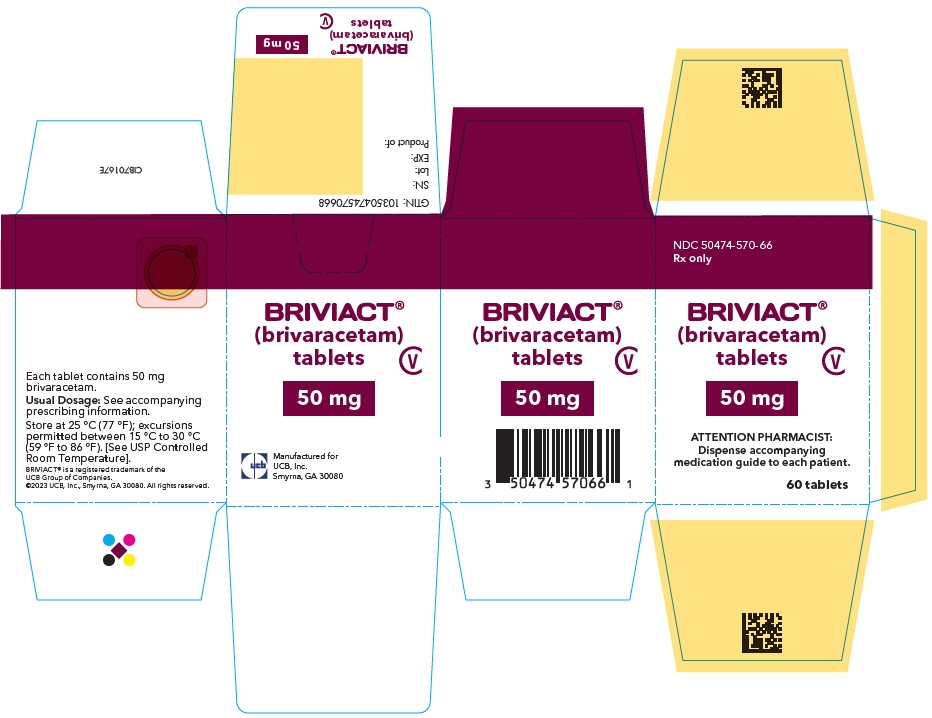
PRINCIPAL DISPLAY PANEL – 50 MG TABLET BOTTLE CARTON
- NDC 50474-570-66
Rx only - BRIVIACT®
(brivaracetam)
tablets - CV
- 50 mg
- ATTENTION PHARMACIST:
Dispense accompanying
medication guide to each patient. - 60 tablets
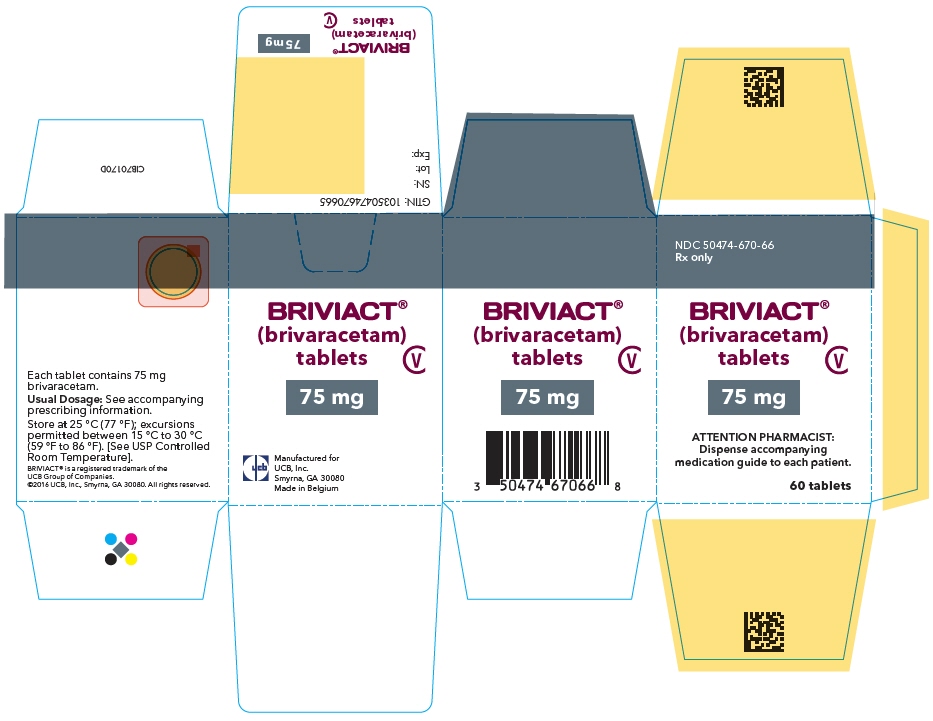
PRINCIPAL DISPLAY PANEL – 75 MG TABLET BOTTLE CARTON
- NDC 50474-670-66
Rx only - BRIVIACT®
(brivaracetam)
tablets - CV
- 75 mg
- ATTENTION PHARMACIST:
Dispense accompanying
medication guide to each patient. - 60 tablets
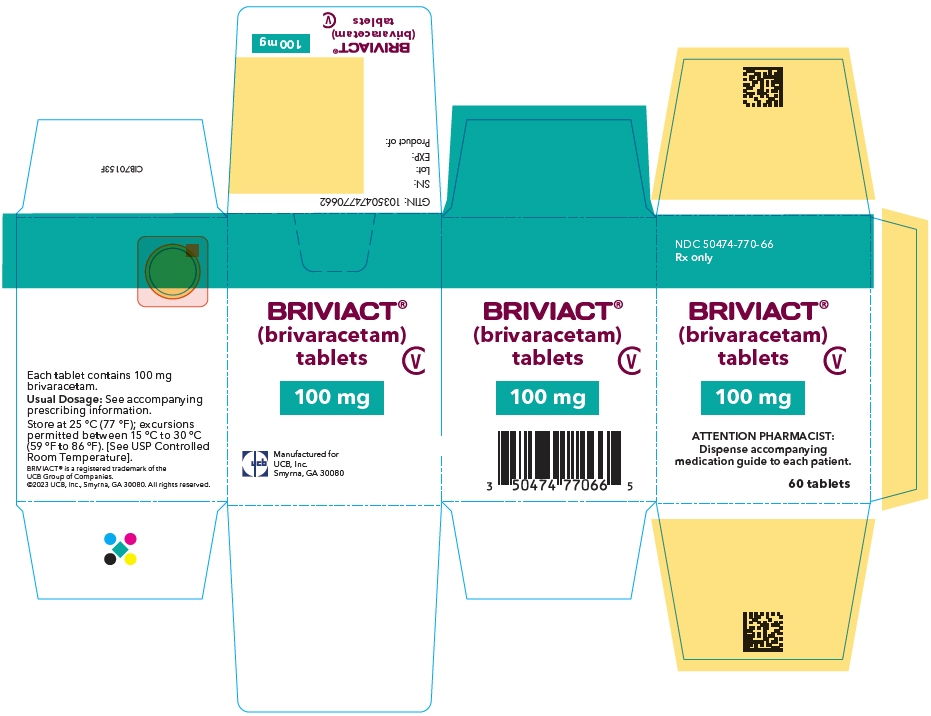
PRINCIPAL DISPLAY PANEL – 100 MG TABLET BOTTLE CARTON
- NDC 50474-770-66
Rx only - BRIVIACT®
(brivaracetam)
tablets - CV
- 100 mg
- ATTENTION PHARMACIST:
Dispense accompanying
medication guide to each patient. - 60 tablets
SRC: NLM .