Bonjesta
Generic name: doxylamine and pyridoxine
Brand names: Bonjesta, Diclegis
Drug class: Miscellaneous antiemetics
Medically reviewed by A Ras MD.
What is Bonjesta?
Bonjesta is a prescription medicine used to treat nausea and vomiting of pregnancy in women who have not improved with change in diet or other non-medicine treatments. It is not known if Bonjesta is safe and effective in women with severe nausea and vomiting of pregnancy, a condition called hyperemesis gravidarum. Women with this condition may need to be hospitalized. It is not known if Bonjesta is safe and effective in children under 18 years of age.
Description
BONJESTA extended-release tablets consist of an enteric-coated core containing 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride, and an immediate release coating of 10 mg doxylamine succinate and 10 mg pyridoxine hydrochloride.
BONJESTA tablets are round, pink, film-coated, multilayer, extended-release tablets containing a total of 20 mg doxylamine succinate and 20 mg pyridoxine hydrochloride. Tablets are imprinted on one side with the pink image of a pregnant woman and a “D” on the other side.
Inactive ingredients are as follows: ammonium hydroxide, n-butanol, carnauba wax powder, colloidal silicon dioxide, croscarmellose sodium, D&C Red#27 aluminum lake, denatured alcohol, ferrosoferric oxide, FD&C Blue #2 aluminum lake, hypromellose, iron oxide red, isopropyl alcohol, magnesium stearate, magnesium trisilicate, methacrylic acid copolymer, microcrystalline cellulose 102, PEG 3350, propylene glycol, shellac glaze, simethicone, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate.
BONJESTA is certified Kosher, Kosher for Passover  and Halal
and Halal  .
.
Doxylamine Succinate
Doxylamine succinate is classified as an antihistamine. The chemical name for doxylamine succinate is ethanamine, N,N-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]-, butanedioate (1:1). The empirical formula is C17H22N2O • C4H6O4 and the molecular mass is 388.46. The structural formula is:
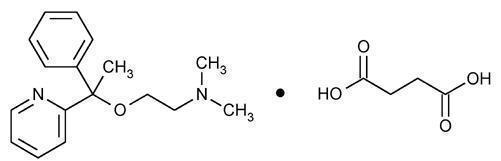
Doxylamine succinate is a white to creamy white powder that is very soluble in water and alcohol, freely soluble in chloroform and very slightly soluble in ether and benzene.
Pyridoxine Hydrochloride
Pyridoxine hydrochloride is a vitamin B6 analog. The chemical name for pyridoxine hydrochloride is 3,4-pyridinedimethanol, 5-hydroxy-6-methyl-, hydrochloride. The empirical formula is C8H11NO3 • HCl and the molecular mass is 205.64. The structural formula is:
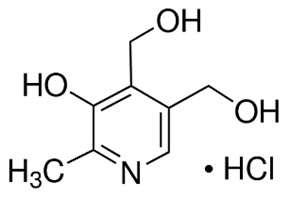
Pyridoxine hydrochloride is a white or practically white crystalline powder that is freely soluble in water, slightly soluble in alcohol and insoluble in ether.
Mechanism of Action
The mechanism of action of BONJESTA is unknown.
Who should not take Bonjesta?
Do not take Bonjesta if you:
- are allergic to doxylamine succinate, other ethanolamine derivative antihistamines, pyridoxine hydrochloride or any of the ingredients in Bonjesta. See the end of this Patient Information leaflet for a complete list of ingredients in Bonjesta.
- take monoamine oxidase inhibitors (MAOIs). Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including Marplan, Nardil, Emsam, Eldepryl, Zelapar, and Parnate.
What should I tell my healthcare provider before taking Bonjesta?
Before taking Bonjesta, tell your healthcare provider about all of your medical conditions, including if you:
- have asthma.
- have eye problems called increased intraocular pressure or narrow angle glaucoma.
- have a stomach problem called stenosing peptic ulcer or pyloroduodenal obstruction.
- have a bladder problem called urinary bladder-neck obstruction.
- are breastfeeding or plan to breastfeed. Bonjesta can pass into your breast milk and may harm your baby. You should not breastfeed while using Bonjesta.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take Bonjesta?
- Talk to your healthcare provider about how much Bonjesta to take and when to take it.
- Take Bonjesta everyday as prescribed by your healthcare provider. Do not stop taking Bonjesta without talking to your healthcare provider first.
- See the following schedule for the right way you should start taking Bonjesta:
- Start with 1 tablet by mouth at bedtime. If your nausea and vomiting is better or controlled on Day 2, continue to take 1 tablet each day at bedtime.
- If you still have nausea and vomiting on Day 2, start taking 1 tablet in the morning and 1 tablet at bedtime each day.
- Do not take more than 2 tablets (1 in the morning and 1 at bedtime) each day.
- Take Bonjesta on an empty stomach with a glass of water.
- Take Bonjesta tablets whole. Do not crush, chew, or break Bonjesta tablets before swallowing. If you cannot swallow Bonjesta tablets whole, tell your healthcare provider.
- If you take too much Bonjesta (overdose), you may have the following symptoms: restlessness, dry mouth, the pupils of your eyes become larger (dilated), sleepiness, dizziness, confusion, fast heart rate, seizures, muscle pain or weakness, urination changes and build-up of fluid in the body. If you have these symptoms and they are severe, they may lead to death. If you take too much Bonjesta, call your poison control center at 1-800-222-1222.
What are the possible side effects of Bonjesta?
Bonjesta may cause serious side effects, including drowsiness.
Drowsiness is a common side effect when taking Bonjesta, but can also be severe:
- Do not drive, operate heavy machinery, or do other activities that need your full attention unless your healthcare provider says that you may do so.
- Do not drink alcohol, or take other central nervous system depressants such as cough and cold medicines, certain pain medicines, and medicines that help you sleep while you take Bonjesta. Severe drowsiness can happen or become worse causing falls or accidents.
Bonjesta may cause false positive urine drug screening test for methadone, opiates and PCP.
These are not all the possible side effects of Bonjesta.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Bonjesta
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. You can ask your pharmacist or healthcare provider for information about Bonjesta that is written for health professionals. Do not use Bonjesta for a condition for which it was not prescribed. Do not give Bonjesta to other people, even if they have the same symptoms that you have. It may harm them.
How should I store Bonjesta?
- Store Bonjesta at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep the bottle tightly closed to protect Bonjesta from moisture.
- The Bonjesta bottle contains a desiccant canister to help keep your medicine dry. Do not throw away the desiccant.
- Safely throw away medicine that is past the expiration date or no longer needed.
Keep Bonjesta and all medicines out of the reach of children.
What are the ingredients in Bonjesta?
Active ingredient: doxylamine succinate (an antihistamine) and pyridoxine hydrochloride (vitamin B6).
Inactive ingredients: ammonium hydroxide, n-butanol, carnauba wax powder, colloidal silicon dioxide, croscarmellose sodium, D&C Red#27 aluminum lake, denatured alcohol, ferrosoferric oxide, FD&C Blue #2 aluminum lake, hypromellose, iron oxide red, isopropyl alcohol, magnesium stearate, magnesium trisilicate, methacrylic acid copolymer, microcrystalline cellulose 102, PEG 3350, propylene glycol, shellac glaze, simethicone, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate.
Label
PACKAGE/LABEL DISPLAY PANEL
- Bottle Label-Outside Front Cover with Imprint Area for Lot & Expiry
- NDC 55494-120-60
- Bonjesta®
(doxylamine succinate and pyridoxine hydrochloride)
Extended-release tablets 20mg/20mg - WARNING: Swallow tablets whole.
Do not crush, chew, or split the tablets. - 60 extended-release tablets
- DUCHESNAY
- Rx only

SRC: NLM .
