Blenrep
Generic name: belantamab mafodotin
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Blenrep?
Blenrep is a prescription medicine used to treat adults with multiple myeloma who have received at least 4 prior medicines to treat multiple myeloma, and their cancer has come back or did not respond to prior treatment.
It is not known if Blenrep is safe and effective in children.
Description
Belantamab mafodotin-blmf is a B-cell maturation antigen (BCMA)-directed antibody and microtubule inhibitor conjugate. Belantamab mafodotin-blmf is an antibody conjugate composed of 3 components: 1) afucosylated, humanized immunoglobulin G1 monoclonal antibody covalently linked to 2) the microtubule inhibitor MMAF via 3) a protease-resistant maleimidocaproyl linker.
The antibody is produced in a mammalian cell line (Chinese Hamster Ovary) using recombinant DNA technology and the microtubule inhibitor and linker are produced by chemical synthesis. Approximately 4 molecules of mafodotin are attached to each antibody molecule. The molecular weight of belantamab mafodotin-blmf is approximately 152 kDa. Belantamab mafodotin-blmf has the following structure:
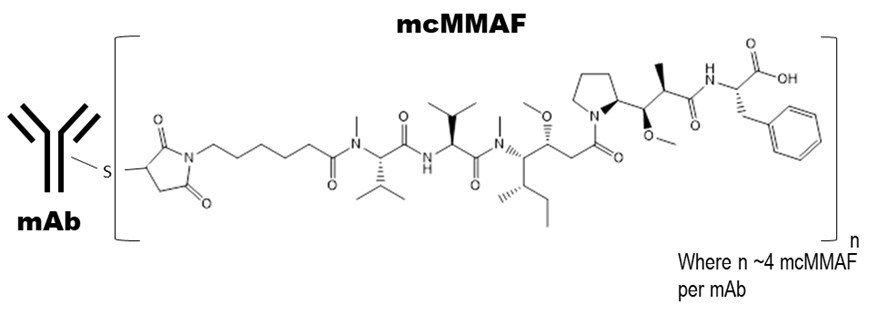
BLENREP (belantamab mafodotin-blmf) for injection is a sterile, preservative-free, white to yellow, lyophilized powder in a single-dose vial for reconstitution and further dilution prior to intravenous use. BLENREP is supplied as 100 mg per vial and requires reconstitution with 2 mL of Sterile Water for Injection, USP, to obtain a concentration of 50 mg/mL. Each mL of reconstituted solution contains belantamab mafodotin-blmf (50 mg) and the inactive ingredients, citric acid (0.42 mg), disodium edetate dihydrate (0.019 mg), polysorbate 80 (0.2 mg), trehalose dihydrate (75.6 mg), and trisodium citrate dihydrate (6.7 mg). The pH of the reconstituted solution is 6.2.
Mechanism of Action
Belantamab mafodotin-blmf is an antibody-drug conjugate (ADC). The antibody component is an afucosylated IgG1 directed against BCMA, a protein expressed on normal B lymphocytes and multiple myeloma cells. The small molecule component is MMAF, a microtubule inhibitor. Upon binding to BCMA, belantamab mafodotin-blmf is internalized followed by release of MMAF via proteolytic cleavage. The released MMAF intracellularly disrupts the microtubule network, leading to cell cycle arrest and apoptosis.
Belantamab mafodotin-blmf had antitumor activity in multiple myeloma cells and mediated killing of tumor cells through MMAF-induced apoptosis, as well as by tumor cell lysis through antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
What is the most important information I should know about Blenrep?
Before you receive Blenrep, you must read and agree to all of the instructions in the Blenrep REMS. Before prescribing Blenrep, your healthcare provider will explain the Blenrep REMS to you and have you sign the Patient Enrollment Form.
Blenrep can cause serious side effects, including:
- Eye problems. Eye problems are common with Blenrep. Blenrep can cause changes to the surface of your eye that can lead to dry eyes, blurred vision, worsening vision, severe vision loss, and corneal ulcer. Tell your healthcare provider if you have any vision changes or eye problems during treatment with Blenrep.
- Your healthcare provider will send you to an eye specialist to check your eyes before you start treatment with Blenrep, prior to each dose of Blenrep, and for worsening symptoms of eye problems.
- Even if your vision seems fine, it is important that you get your eyes checked during treatment with Blenrep because some changes can happen without symptoms and may only be seen on an eye exam.
- You should use preservative-free lubricant eye drops at least 4 times per day during treatment with Blenrep as instructed by your healthcare provider.
- You should use caution when driving or operating machinery as Blenrep may affect your vision.
- Avoid wearing contact lenses during treatment with Blenrep unless directed by your eye specialist.
See “What are the possible side effects of Blenrep?” for more information about serious side effects.
What should I tell my healthcare provider before using Blenrep?
Before receiving Blenrep, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of vision or eye problems.
- have bleeding problems or a history of bleeding problems.
- are pregnant or plan to become pregnant. Blenrep can harm your unborn baby.
Females who are able to become pregnant:- Your healthcare provider may do a pregnancy test before you start treatment with Blenrep.
- You should use effective birth control during treatment with Blenrep and for 4 months after the last dose. Talk to your healthcare provider about birth control methods you can use during this time.
- Tell your healthcare provider if you become pregnant or think you may be pregnant during treatment with Blenrep.
- Males with female partners who are able to become pregnant should use effective birth control during treatment with Blenrep and for 6 months after the last dose.
- are breastfeeding or plan to breastfeed. It is not known if Blenrep passes into your breast milk. Do not breastfeed during treatment with Blenrep and for 3 months after the last dose.
- Blenrep may affect fertility in males and females. Talk to your healthcare provider if this is a concern for you.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Blenrep?
- Blenrep will be given to you by your healthcare provider by intravenous infusion into your vein over approximately 30 minutes.
- Blenrep is usually given every 3 weeks.
- Your healthcare provider will decide how many treatments you need.
- Your healthcare provider may decrease your dose, temporarily stop or completely stop treatment with Blenrep if you have serious side effects.
- If you miss any appointments, call your healthcare provider as soon as possible to reschedule your appointment.
What are the possible side effects of Blenrep?
Blenrep can cause serious side effects, including:
- See Eye Problems in “What is the most important information I should know about Blenrep?”
- Decrease in platelets (thrombocytopenia) is common with Blenrep, and can also be serious. Platelets are a type of blood cell that help your blood to clot. Your healthcare provider will check your blood cell counts before you start treatment with Blenrep and during treatment. Tell your healthcare provider if you have bleeding or bruising during treatment with Blenrep.
- Infusion reactions are common with Blenrep, and can also be serious. Tell your healthcare provider or nurse right away if you get any of the following signs or symptoms of an infusion reaction while receiving Blenrep:
- chills or shaking
- redness of your face (flushing)
- itching or rash
- shortness of breath, cough, or wheezing
- swelling of your lips, tongue, throat, or face
- dizziness
- feel like passing out
- tiredness
- fever
- feel like your heart is racing (palpitations)
- The most common side effects of Blenrep include vision or eye changes such as findings on eye exam (keratopathy), decreased vision or blurred vision, nausea, low blood cell counts, fever, infusion-related reactions, tiredness, and changes in kidney or liver function blood tests.
These are not all the possible side effects of Blenrep.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Blenrep
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about Blenrep that is written for health professionals.
What are the ingredients in Blenrep?
Active Ingredient: belantamab mafodotin-blmf
Inactive Ingredients: citric acid, disodium edetate dihydrate, polysorbate 80, trehalose dihydrate, trisodium citrate dihydrate.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0173-0896-01
- BLENREP
- (belantamab mafodotin-blmf)
- for injection
- 100 mg/vial
- CAUTION: Hazardous Agent.
- For intravenous infusion after reconstitution and dilution.
- Single-dose vial.
- Discard unused portion.
- No preservative.
- No U.S. standard of potency.
- Dispense the enclosed Medication Guide to each patient.
- ©2020 GSK group of companies or its licensor.
- Rev. 8/20
- 62000000041460

SRC: NLM .
