Bevespi Aerosphere
Generic name: formoterol and glycopyrrolate
Drug class: Bronchodilator combinations
Medically reviewed by A Ras MD.
What is Bevespi Aerosphere?
Bevespi Aerosphere combines an anticholinergic, glycopyrrolate, and a long-acting beta2-adrenergic agonist (LABA) medicine, formoterol fumarate.
Anticholinergic and LABA medicines help the muscles around the airways in your lungs stay relaxed to prevent symptoms such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe. Bevespi Aerosphere is a prescription medicine used to treat COPD. COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both.
Bevespi Aerosphere is used long term as 2 inhalations, 2 times each day in the morning and in the evening, to improve symptoms of COPD for better breathing. Bevespi Aerosphere is not for use to treat sudden symptoms of COPD. Always have a rescue inhaler (an inhaled, short-acting bronchodilator) with you to treat sudden symptoms. If you do not have a rescue inhaler, contact your healthcare provider to have one prescribed for you. Bevespi Aerosphere is not for the treatment of asthma. It is not known if Bevespi Aerosphere is safe and effective in people with asthma. Bevespi Aerosphere should not be used in children. It is not known if Bevespi Aerosphere is safe and effective in children.
Description
BEVESPI AEROSPHERE (glycopyrrolate and formoterol fumarate) Inhalation Aerosol is a pressurized metered-dose inhaler that contains a combination of micronized glycopyrrolate, an anticholinergic, and micronized formoterol fumarate, a long-acting beta2-adrenergic agonist, for oral inhalation.
Glycopyrrolate is a quaternary ammonium salt with the following chemical name: (RS)-[3-(SR)-Hydroxy-1,1-dimethylpyrrolidinium bromide] α-cyclopentylmandelate. Glycopyrrolate is a powder that is freely soluble in water. The molecular formula is C19H28BrNO3, and the molecular weight is 398.33 g/mol. The structural formula is as follows:
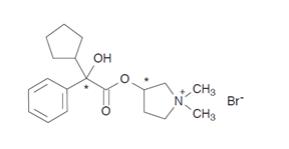
Glycopyrrolate contains two chiral centers (denoted by * in structure above) and is a racemate of a 1:1 mixture of the R,S and S,R diastereomers. The active moiety, glycopyrronium, is the positively charged ion of glycopyrrolate.
Formoterol fumarate has the chemical name N-[2-Hydroxy-5-[(1RS)-1-hydroxy-2-[[(1RS)-2-(4-methoxyphenyl)-1- methylethyl]-amino] ethyl]phenyl] formamide, €-2-butenedioate dihydrate. Formoterol fumarate is a powder that is slightly soluble in water. The molecular formula is (C19H24N2O4)2.C4H4O4.2H2O and the molecular weight is 840.91 g/mol. The structural formula is as follows:
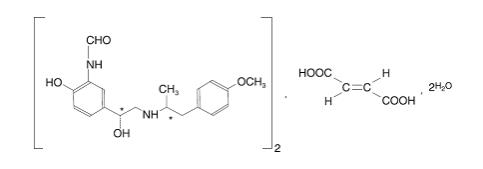
Formoterol fumarate contains two chiral centers (denoted by * in structure above), and consists of a single enantiomeric pair (a racemate of R,R and S,S).
BEVESPI AEROSPHERE is formulated as a hydrofluoroalkane (HFA 134a) propelled pressurized metered dose inhaler containing 28 or 120 inhalations. The canister has an attached dose indicator and is supplied with a white plastic actuator body and mouthpiece with an orange dust cap.
After priming each actuation of the inhaler meters 10.4 mcg of glycopyrrolate (equivalent to 8.3 mcg of glycopyrronium) and 5.5 mcg of formoterol fumarate from the valve which delivers 9 mcg of glycopyrrolate (equivalent to 7.2 mcg of glycopyrronium) and 4.8 mcg of formoterol fumarate from the actuator. The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between actuation of the device and inspiration through the delivery system. BEVESPI AEROSPHERE also contains porous particles that form a cosuspension with the drug crystals. The porous particles are comprised of the phospholipid, 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC), and calcium chloride. Porous particles and HFA 134a are excipients in the formulation.
Mechanism of Action
BEVESPI AEROSPHERE
BEVESPI AEROSPHERE contains both glycopyrrolate and formoterol fumarate. The mechanism of action described below for the individual components applies to BEVESPI AEROSPHERE. These drugs represent two different classes of medications (an anticholinergic, and a long-acting selective beta2-adrenoceptor agonist) that have different effects on clinical physiology and inflammatory indices of COPD.
Glycopyrrolate
Glycopyrrolate is a long-acting antimuscarinic agent which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of the M3 receptor at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methylcholine and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted more than 12 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of glycopyrrolate is predominantly a site-specific effect.
Formoterol Fumarate
Formoterol fumarate is a long-acting selective beta2-adrenergic agonist (beta2-agonist) with a rapid onset of action. Inhaled formoterol fumarate acts locally in the lung as a bronchodilator. In vitro studies have shown that formoterol has more than 200-fold greater agonist activity at beta2-receptors than at beta1-receptors. The in vitro binding selectivity to beta2– over beta1-adrenoceptors is higher for formoterol than for albuterol (5 times), whereas salmeterol has a higher (3 times) beta2-selectivity ratio than formoterol.
Although beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-receptors are the predominant receptors in the heart, there are also beta2-receptors in the human heart comprising 10% to 50% of the total beta-adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenoceptor agonist drugs, including formoterol fumarate, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3′, 5′-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
Who should not take Bevespi Aerosphere?
Do not use Bevespi Aerosphere if you:
- are allergic to glycopyrrolate, formoterol fumarate, or to any of the ingredients in Bevespi Aerosphere. See the end of this Patient Information leaflet for a complete list of ingredients.
- have asthma.
What should I tell my healthcare provider before taking Bevespi Aerosphere?
Before using Bevespi Aerosphere, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems
- have high blood pressure
- have seizures
- have thyroid problems
- have diabetes
- have liver problems
- have eye problems such as glaucoma. Bevespi Aerosphere may make your glaucoma worse.
- have prostate or bladder problems, or problems passing urine. Bevespi Aerosphere may make these problems worse.
- are allergic to any other medicines or food products
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if Bevespi Aerosphere may harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if the medicines, glycopyrrolate and formoterol fumarate, in Bevespi Aerosphere pass into your breast milk and if they can harm your baby.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Bevespi Aerosphere and certain other medicines may interact with each other. This may cause serious side effects.
Especially tell your healthcare provider if you take:
- anticholinergics (including tiotropium, ipratropium, aclidinium, and umeclidinium)
- other LABAs (including salmeterol, arformoterol, vilanterol, olodaterol, and indacaterol)
- atropine
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine.
How should I take Bevespi Aerosphere?
Read the step-by-step instructions that comes with Bevespi Aerosphere.
- Do not use Bevespi Aerosphere unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- Use Bevespi Aerosphere exactly as prescribed. Do not use Bevespi Aerosphere more often than prescribed.
- Use 2 inhalations of Bevespi Aerosphere, 2 times each day (in the morning and in the evening).
- If you miss a dose of Bevespi Aerosphere, take your next dose at the same time you normally do. Do not take more than your prescribed dose of Bevespi Aerosphere.
- If you take too much Bevespi Aerosphere, call your healthcare provider or go to the nearest hospital emergency room right away if you have unusual symptoms, such as worsening shortness of breath, chest pain, increased heart rate, or shakiness.
- Do not spray Bevespi Aerosphere in your eyes. If Bevespi Aerosphere gets in your eyes, rinse them well with water. If redness continues, call your healthcare provider.
- Do not stop using Bevespi Aerosphere unless told to do so by your healthcare provider because your symptoms might come back. Your healthcare provider will change your medicines as needed.
- Do not use other medicines that contain a LABA or an anticholinergic for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are LABA or anticholinergic containing medicines.
- Bevespi Aerosphere does not relieve sudden symptoms of COPD. Always have a rescue inhaler with you to treat sudden symptoms. If you do not have a rescue inhaler, call your healthcare provider to have one prescribed for you.
- Call your healthcare provider or get medical care right away if your breathing problems get worse; you need to use your rescue inhaler more often than usual, or your rescue inhaler does not work as well to relieve your symptoms.
What are the possible side effects of Bevespi Aerosphere?
Bevespi Aerosphere can cause serious side effects, including:
- people with asthma who take LABA medicines, such as formoterol fumarate (one of the medicines in Bevespi Aerosphere), without also using a medicine called an inhaled corticosteroid, have an increased risk of serious problems from asthma, including being hospitalized, needing a tube placed in their airway to help them breathe, or death.
- Call your healthcare provider if breathing problems worsen over time while using Bevespi Aerosphere. You may need a different treatment.
- Get emergency medical care if:
- your breathing problems worsen quickly
- you use your rescue inhaler medicine, but it does not relieve your breathing problems
- using too much of a LABA medicine may cause:
- chest pain
- fast and irregular heartbeat
- tremor
- increased blood pressure
- headache
- nervousness
- COPD symptoms can get worse over time. If your COPD symptoms worsen over time, do not increase your dose of Bevespi Aerosphere, instead call your healthcare provider.
- sudden breathing problems immediately after inhaling your medicine. If you have sudden breathing problems immediately after inhaling your medicine, stop using Bevespi Aerosphere and call your healthcare provider right away.
- serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction:
- rash
- hives
- swelling of the face, mouth, and tongue
- breathing problems
- effects on your heart:
- increase blood pressure
- a fast or irregular heartbeat
- chest pain
- effects on your nervous system:
- tremor
- nervousness
- changes in laboratory blood levels, including high levels of blood sugar (hyperglycemia) and low levels of potassium (hypokalemia) which may cause symptoms of muscle spasm, muscle weakness or abnormal heart rhythm.
- new or worsened eye problems including acute narrow-angle glaucoma. Acute narrow-angle glaucoma can
- cause permanent loss of vision if not treated. Symptoms of acute narrow-angle glaucoma may include:
- urinary retention. People who take Bevespi Aerosphere may develop new or worse urinary retention. Symptoms of urinary retention may include:
- difficulty urinating
- painful urination
- urinating frequently
- urination in a weak stream or dripsIf you have these symptoms of urinary retention, stop taking Bevespi Aerosphere and call your healthcare provider right away before taking another dose.
Common side effects of Bevespi Aerosphere include: urinary tract infection and cough.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of Bevespi Aerosphere. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to AstraZeneca at 1-800-236-9933.
General information about the safe and effective use of Bevespi Aerosphere
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Bevespi Aerosphere for a condition for which it was not prescribed. Do not give your Bevespi Aerosphere to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Bevespi Aerosphere that is written for health professionals.
How should I store Bevespi Aerosphere?
- Store Bevespi Aerosphere at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
- Do not put a hole in the Bevespi Aerosphere canister.
- Do not use or store Bevespi Aerosphere near heat or a flame. Temperatures above 120ºF (49ºC) may cause the canister to burst.
- Do not throw the Bevespi Aerosphere canister into a fire or an incinerator.
- Throw away Bevespi Aerosphere 3 months after you open the foil pouch (3 weeks for the 28 inhalation canister) or when the dose indicator reaches zero “0”, whichever comes first.
- Keep Bevespi Aerosphere and all medicines out of the reach of children.
What are the ingredients in Bevespi Aerosphere?
Active ingredients: micronized glycopyrrolate and micronized formoterol fumarate
Inactive ingredients: hydrofluoroalkane (HFA 134a) and porous particles (comprised of DSPC [1,2-Distearoyl-sn-glycero-3-phosphocholine] and calcium chloride).
Label
PRINCIPAL DISPLAY PANEL – 9 MCG/4.8 MCG PER INHALATION
- NDC 0310-4600-39
BEVESPI - AEROSPHERE®
- (glycopyrrolate and formoterol
- fumarate) Inhalation Aerosol
9 mcg/4.8 mcg per inhalation - For Oral Inhalation only
- RX only
- Shake inhaler well before using
- Inhalation Aerosol
- 28 inhalations
- AstraZeneca

SRC: NLM .
