Benlysta
Generic name: belimumab
Drug class: Selective immunosuppressants
Medically reviewed by A Ras MD.
What is Benlysta?
Benlysta is a prescription medicine used to treat patients with active systemic lupus erythematosus (SLE or lupus) who are receiving other lupus medicines. Benlysta is also used to treat adult patients with active lupus nephritis (lupus-related kidney inflammation) who are receiving other lupus medicines.
Both intravenous and subcutaneous dosing of Benlysta are approved for adults with SLE and lupus nephritis.
Intravenous dosing of Benlysta is approved in children aged 5 years and older with SLE.
Benlysta contains belimumab which is in a group of medicines called monoclonal antibodies. Lupus is a disease of the immune system (the body system that fights infection). When given together with other medicines for lupus, Benlysta decreases lupus disease activity more than other lupus medicines alone.
- It is not known if Benlysta is safe and effective in people with severe active central nervous system lupus.
Description
Belimumab is a human IgG1λ monoclonal antibody specific for soluble human B lymphocyte stimulator protein (BLyS, also referred to as BAFF and TNFSF13B). Belimumab has a molecular weight of approximately 147 kDa. Belimumab is produced by recombinant DNA technology in a murine cell (NS0) expression system.
Intravenous Infusion
BENLYSTA (belimumab) for injection is a sterile, white to off-white, preservative‑free, lyophilized powder in a single-dose vial for reconstitution and dilution prior to intravenous infusion. BENLYSTA for injection is supplied as 120 mg per vial and 400 mg per vial and requires reconstitution with Sterile Water for Injection, USP (1.5 mL and 4.8 mL, respectively) to obtain a concentration of 80 mg/mL [see Dosage and Administration (2.2)]. After reconstitution, each vial allows for withdrawal of 1.5 mL (120 mg) or 5 mL (400 mg). Each mL delivers 80 mg belimumab, citric acid (0.16 mg), polysorbate 80 (0.4 mg), sodium citrate (2.7 mg), and sucrose (80 mg), with a pH of 6.5.
The vial stoppers are not made with natural rubber latex.
Subcutaneous Injection
BENLYSTA (belimumab) injection is a sterile, preservative-free, clear to opalescent, and colorless to pale yellow solution for subcutaneous use. It is supplied in a 1-mL single-dose prefilled autoinjector with a fixed 27-gauge, half-inch needle or in a 1-mL single-dose prefilled syringe with a fixed 27-gauge, half-inch needle with a needle guard. Each 1 mL delivers 200 mg belimumab, L-arginine hydrochloride (5.3 mg), L-histidine (0.65 mg), L-histidine monohydrochloride (1.2 mg), polysorbate 80 (0.1 mg), and sodium chloride (6.7 mg), with a pH of 6.0.
The autoinjectors and prefilled syringes are not made with natural rubber latex.
Mechanism of Action
BENLYSTA is a BLyS-specific inhibitor that blocks the binding of soluble BLyS, a B-cell survival factor, to its receptors on B cells. BENLYSTA does not bind B cells directly, but by binding BLyS, BENLYSTA inhibits the survival of B cells, including autoreactive B cells, and reduces the differentiation of B cells into immunoglobulin-producing plasma cells.
What is the most important information I should know about Benlysta?
Immunosuppressive agents, including Benlysta can cause serious side effects. Some of these side effects may cause death. The following are serious adverse reactions that have occurred in patients receiving Benlysta:
1. Infections. Infections could be serious, leading to hospitalization or death. Tell your healthcare provider right away if you have any of the following symptoms of an infection:
- fever
- chills
- pain or burning with urination
- urinating often
- coughing up mucus
- warm, red, or painful skin or sores on your body
2. Allergic (hypersensitivity) reactions. Serious allergic reactions can happen on the day of, or in the days after, receiving Benlysta and may cause death. Your healthcare provider will watch you closely while you are receiving Benlysta given intravenously (infusion) and after your infusion for signs of a reaction. Allergic reactions can sometimes be delayed. Tell your healthcare provider right away if you have any of the following symptoms of an allergic reaction following use of Benlysta:
- itching
- swelling of the face, lips, mouth, tongue, or throat
- trouble breathing
- anxiousness
- low blood pressure
- dizziness or fainting
- headache
- nausea
- skin rash
3. Mental health problems and suicide. Symptoms of mental health problems can occur. Tell your healthcare provider right away if you have any of the following symptoms:
- thoughts of suicide or dying
- attempt to commit suicide
- trouble sleeping (insomnia)
- new or worse anxiety
- new or worse depression
- acting on dangerous impulses
- other unusual changes in your behavior or mood
- thoughts of hurting yourself or others
Who should not use Benlysta?
Do not use li if you:
- are allergic to belimumab or any of the ingredients in Benlysta. See the end of this Medication Guide for a complete list of ingredients in Benlysta.
What should I tell my healthcare provider before using Benlysta?
Before you receive Benlysta, tell your healthcare provider about all of your medical conditions, including if you:
- think you have an infection or have infections that keep coming back. You should not receive Benlysta if you have an infection unless your healthcare provider tells you to. See “What is the most important information I should know about Benlysta?”
- have or have had mental health problems such as depression or thoughts of suicide.
- have recently received a vaccination or if you think you may need a vaccination. If you are receiving Benlysta, you should not receive live vaccines.
- are allergic to other medicines.
- are receiving other biologic medicines or monoclonal antibodies.
- have or have had any type of cancer.
- are pregnant or plan to become pregnant. It is not known if Benlysta will harm your unborn baby. You should talk to your healthcare provider about whether to prevent pregnancy while on Benlysta. If you choose to prevent pregnancy, you should use an effective method of birth control while receiving Benlysta and for at least 4 months after the final dose of Benlysta.
- Tell your healthcare provider right away if you become pregnant during your treatment with Benlysta or if you think you may be pregnant.
- If you become pregnant while receiving Benlysta, talk to your healthcare provider about enrolling in the Benlysta Pregnancy Registry. You can enroll in this registry by calling 1-877-681-6296. The purpose of this registry is to monitor the health of you and your baby.
- are breastfeeding or plan to breastfeed. It is not known if Benlysta passes into your breast milk. You and your healthcare provider should talk about whether or not you should receive Benlysta and breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of your medicines with you to show to your healthcare provider and pharmacist when you get a new medicine.
How should I use Benlysta?
When administered intravenously (by vein)
- You will be given Benlysta by a healthcare provider through a needle placed in a vein (IV infusion). It takes about 1 hour to give you the full dose of Benlysta.
- Your healthcare provider will tell you how often you should receive Benlysta.
- Your healthcare provider may give you medicines before you receive Benlysta to help reduce your chance of having a reaction. A healthcare provider will watch you closely while you are receiving Benlysta and after your infusion for signs of a reaction. A healthcare provider will review the signs and symptoms of possible allergic reactions that could happen after your infusion.
When administered subcutaneously (under your skin)
- Your healthcare provider will tell you how often you should use Benlysta. Use Benlysta exactly as your healthcare provider tells you to.
- Read the Instructions for Use that comes with Benlysta for instructions about the right way to give your injections at home.
- Benlysta may be prescribed as a single-dose autoinjector or as a single-dose prefilled syringe.
- Before you use Benlysta, your healthcare provider will show you or your caregiver how to give the injections and review the signs and symptoms of possible allergic reactions.
- Benlysta is injected under your skin (subcutaneously) of your stomach (abdomen) or thigh.
- Use Benlysta once a week on the same day each week. If you have lupus nephritis, one dose may be 2 injections.
- If you miss your dose of Benlysta on your planned day, inject a dose as soon as you remember. Then, inject your next dose at your regularly scheduled time or continue weekly dosing based on the new day injected. In case you are not sure when to inject Benlysta, call your healthcare provider.
What are the possible side effects of Benlysta?
Benlysta can cause serious side effects, including:
See “What is the most important information I should know about Benlysta?”
- Progressive multifocal leukoencephalopathy (PML). PML is a serious and life-threatening brain infection. Your chance of getting PML may be higher if you are treated with medicines that weaken your immune system, including Benlysta. PML can result in death or severe disability. If you notice any new or worsening medical problems such as those below, tell your healthcare provider right away:
- memory loss
- trouble thinking
- dizziness or loss of balance
- difficulty talking or walking
- loss of vision
- Cancer. Benlysta may reduce the activity of your immune system. Medicines that affect the immune system may increase your risk of certain cancers.
The most common side effects of Benlysta include:
- nausea
- diarrhea
- fever
- stuffy or runny nose and sore throat (nasopharyngitis)
- persistent cough (bronchitis)
- trouble sleeping (insomnia)
- leg or arm pain
- depression
- headache (migraine)
- pain, redness, itching, or swelling at the site of injection (when given subcutaneously)
These are not all the possible side effects of Benlysta. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1‑800‑FDA‑1088.
General information about the safe and effective use of Benlysta
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Benlysta for a condition for which it was not prescribed. Do not give Benlysta to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Benlysta. You can ask your healthcare provider or pharmacist for information about Benlysta that is written for healthcare professionals.
How should I store Benlysta?
- Store autoinjectors and prefilled syringes in the refrigerator at 36°F to 46°F (2°C to 8°C). Do not freeze.
- Keep Benlysta autoinjectors and prefilled syringes in the original package until time of use to protect from light.
- Do not shake. Keep away from heat and sunlight.
- Do not use and do not place back in the refrigerator if left out at room temperature for more than 12 hours.
- Safely throw away medicine that is out of date or no longer needed.
Keep Benlysta and all medicines out of the reach of children.
What are the ingredients in Benlysta?
Active ingredient: belimumab.
Inactive ingredients:
Intravenous: citric acid, polysorbate 80, sodium citrate, sucrose.
Subcutaneous: L-arginine hydrochloride, L-histidine, L-histidine monohydrochloride, polysorbate 80, sodium chloride.
For more information, go to www.BENLYSTA.com or call 1-877-423-6597
Instructions for use for Benlysta
Benlysta (ben-LIST-ah)
(belimumab)
injection, for subcutaneous use
Prefilled Syringe
Read this Instructions for Use before you start to use Benlysta and each time you refill your prescription. There may be new information. You should also receive training from your healthcare provider on how to use the prefilled syringe the right way. If you do not follow these instructions the prefilled syringe may not work properly. Benlysta is for use under the skin only (subcutaneous).
Important Storage Information
- Keep refrigerated until 30 minutes before use.
- Keep in the original package until time of use to protect from light.
- Do not freeze Benlysta.
- Keep away from heat and sunlight.
- Do not use and do not place back in the refrigerator if Benlysta is left out for more than 12 hours.
- Keep out of the reach of children.
Important Warnings
- The prefilled syringe should be used only 1 time and then thrown away. See below, “Dispose of used prefilled syringe and inspect.”
- Do not share your Benlysta prefilled syringe with other people. You may give other people a serious infection or get a serious infection from them.
- Do not shake the prefilled syringe.
- Do not use if dropped onto a hard surface.
- Do not remove the Needle Cap until right before the injection.
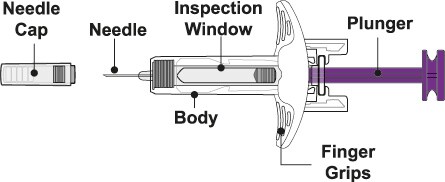
Benlysta Prefilled Syringe Parts
Before use
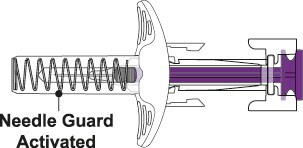
After Use – Needle is covered by Needle Guard
Supplies needed for the injection
Benlysta Prefilled Syringe

Alcohol Swab (not included)
Gauze Pad or Cotton Ball (not included)
Sharps Container (not included)
1 Gather and check supplies
Gather supplies
- Remove 1 sealed tray containing a prefilled syringe from the refrigerator.
- Find a comfortable, well-lit, and clean surface and place the following supplies within reach:
- Benlysta Prefilled Syringe
- Alcohol Swab (not included)
- Gauze Pad or Cotton Ball (not included)
- Sharps Container (not included)
- Do not perform the injection if you do not have all the supplies listed.
Check expiration date
- Peel back the film of the tray and remove the prefilled syringe by holding the middle of the syringe body.
- Check the expiration date on the prefilled syringe. See Figure A.
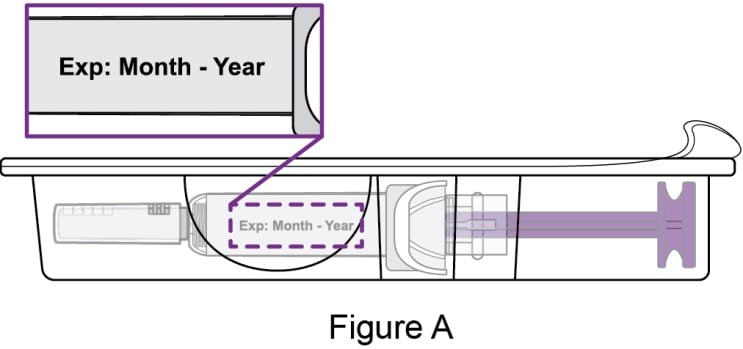
- Do not use if the expiration date has passed.
2 Prepare and inspect the Benlysta Prefilled Syringe
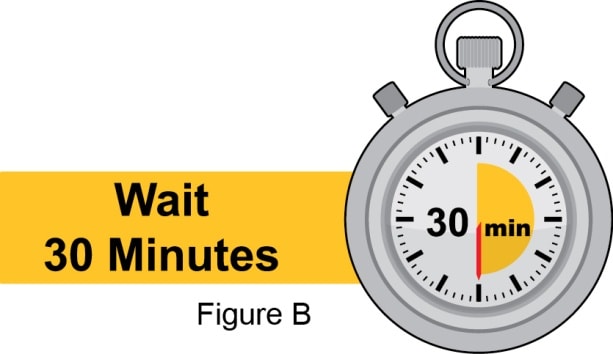
Allow to come to room temperature
- Allow the prefilled syringe to sit at room temperature for 30 minutes. See Figure B.
- Do not warm the prefilled syringe in any other way. For example, do not warm in a microwave oven, hot water, or in direct sunlight.
- Do not remove the Needle Cap during this step.
Inspect Benlysta solution
- Look in the Inspection Window to check that the Benlysta solution is colorless to slightly yellow in color. See Figure C.
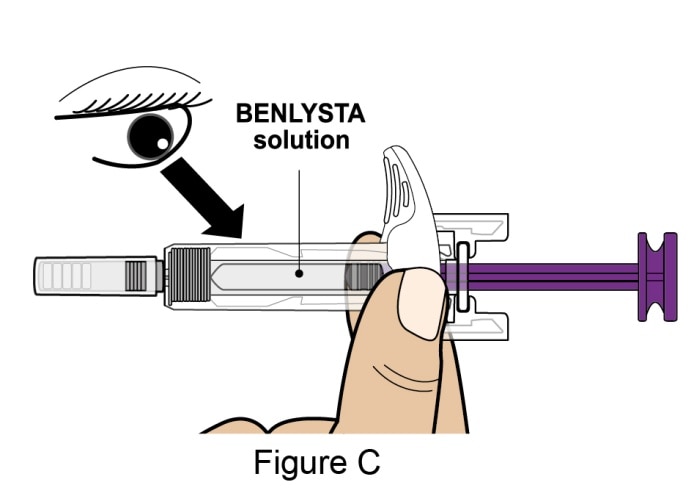
- It is normal to see 1 or more air bubbles in the solution.
- Do not use if the solution looks cloudy, discolored, or has particles.
3 Choose and clean the injection site
Choose the injection site
- Choose where to inject (abdomen or thigh). See Figure D.
- If you need 2 injections to complete your dose, leave at least 2 inches between each injection if using the same site.
- Avoid injecting into the same site each time or in areas where the skin is tender, bruised, red, or hard.
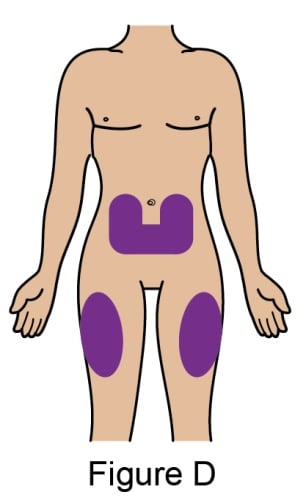
- Do not inject within 2 inches of the belly button.
Clean the injection site
- Wash your hands.
- Clean the injection site by wiping it with an Alcohol Swab. Allow the skin to air dry. See Figure E.
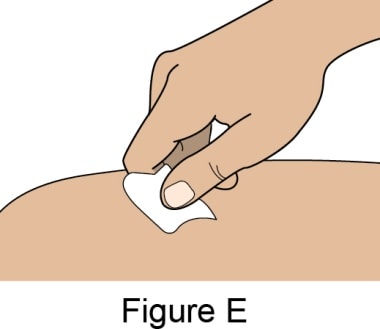
- Do not touch this area again before giving the injection.
4 Prepare for the injection
Remove the Needle Cap
- Do not remove the Needle Cap until right before you give the injection.
- Hold the prefilled syringe by the body and with the Needle facing away from you. Remove the Needle Cap by pulling it straight off. See Figure F.
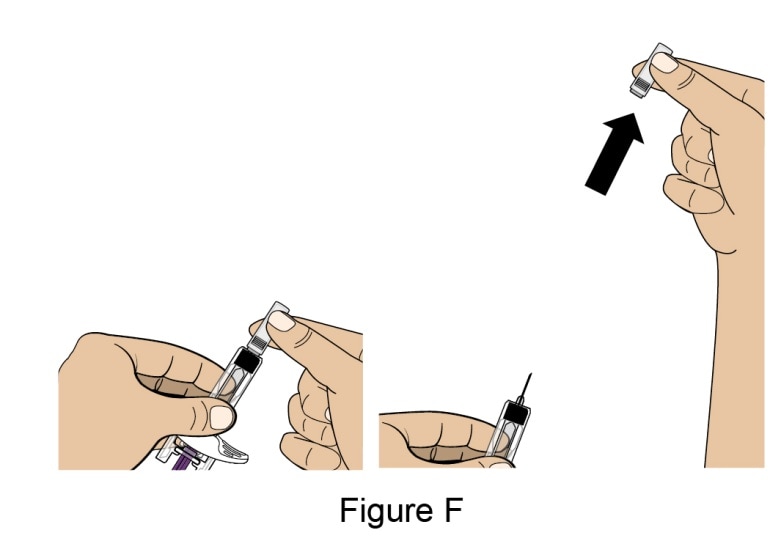
- You may see a drop of liquid at the end of the Needle. This is normal.
- Do not let the Needle touch any surface.
- Do not push any air bubbles out of the prefilled syringe.
- Do not put the Needle Cap back onto the prefilled syringe.
- Keep your hands away from the Plunger to avoid pushing it before injecting.
5 Inject Benlysta
Insert the Needle
- Hold the prefilled syringe in one hand and use your free hand to gently pinch the skin around the injection site. See Figure G.
- Insert the entire Needle into the pinched area of the skin at a slight 45-degree angle using a dart-like motion.
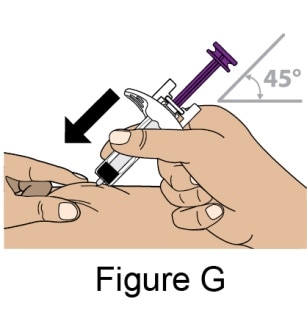
- After the Needle is completely inserted, release the pinched skin.
Complete the injection
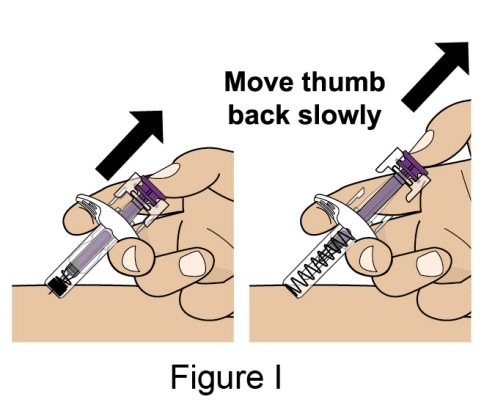
- Push the Plunger all the way down until all of the solution is injected. See Figure H.
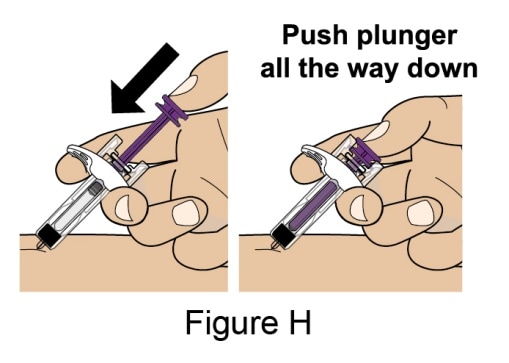
- While keeping your hold on the syringe, slowly move your thumb back, allowing the Plunger to rise up. The Needle will automatically rise up into the Needle Guard. See Figure I.
6 Dispose of used prefilled syringe and inspect
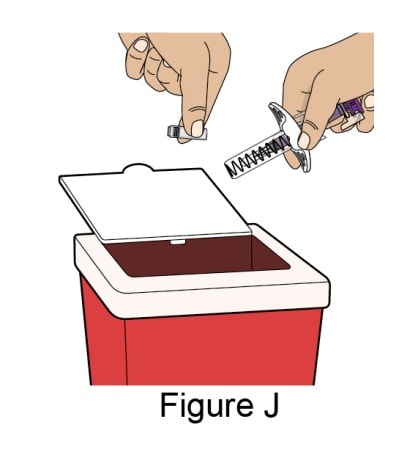
Dispose of the used prefilled syringe
Throw away (dispose of) the used syringe and Needle Cap in a Sharps Container. See Figure J.
- Put your used syringe and sharps in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that:
- is made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- is upright and stable during use,
- is leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to: HTTP://WWW.FDA.GOV/SAFESHARPSDISPOSAL.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Inspect the injection site
- There may be a small amount of blood at the injection site. If needed, press a Cotton Ball or Gauze Pad on the injection site.
- Do not rub the injection site.
Additional information
For more information about Benlysta, go to WWW.Benlysta.COM or call 1-877-423-6597.
Label
PRINCIPAL DISPLAY PANEL
- NDC 49401-088-35
- Benlysta
- (belimumab)
- Injection
- 200 mg/mL
- Rx only
- Once-weekly
- For Subcutaneous Use
- Contents:

