Baraclude
Generic name: entecavir
Drug class: Nucleoside reverse transcriptase inhibitors (NRTIs)
Medically reviewed by A Ras MD.
What is Baraclude?
Baraclude is a prescription medicine used to treat chronic hepatitis B virus (HBV) in adults and children 2 years of age and older who have active liver disease. Baraclude will not cure HBV. Baraclude may lower the amount of HBV in the body. Baraclude may lower the ability of HBV to multiply and infect new liver cells.
Baraclude may improve the condition of your liver. It is not known whether Baraclude will reduce your chances of getting liver cancer or liver damage (cirrhosis), which may be caused by chronic HBV infection. It is not known if Baraclude is safe and effective for use in children less than 2 years of age.
Description
BARACLUDE® is the tradename for entecavir, a guanosine nucleoside analogue with selective activity against HBV. The chemical name for entecavir is 2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one, monohydrate. Its molecular formula is C12H15N5O3∙H2O, which corresponds to a molecular weight of 295.3. Entecavir has the following structural formula:
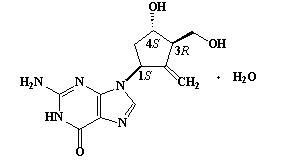
Entecavir is a white to off-white powder. It is slightly soluble in water (2.4 mg/mL), and the pH of the saturated solution in water is 7.9 at 25° C ± 0.5° C.
BARACLUDE film-coated tablets are available for oral administration in strengths of 0.5 mg and 1 mg of entecavir. BARACLUDE 0.5 mg and 1 mg film-coated tablets contain the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, crospovidone, povidone, and magnesium stearate. The tablet coating contains titanium dioxide, hypromellose, polyethylene glycol 400, polysorbate 80 (0.5 mg tablet only), and iron oxide red (1 mg tablet only). BARACLUDE Oral Solution is available for oral administration as a ready-to-use solution containing 0.05 mg of entecavir per milliliter. BARACLUDE Oral Solution contains the following inactive ingredients: maltitol, sodium citrate, citric acid, methylparaben, propylparaben, and orange flavor.
What is the most important information I should know about Baraclude
1. Your hepatitis B virus (HBV) infection may get worse if you stop taking Baraclude. This usually happens within 6 months after stopping Baraclude.
- Take Baraclude exactly as prescribed.
- Do not run out of Baraclude.
- Do not stop Baraclude without talking to your healthcare provider.
- Your healthcare provider should monitor your health and do regular blood tests to check your liver if you stop taking Baraclude.
2. If you have or get HIV that is not being treated with medicines while taking Baraclude, the HIV virus may develop resistance to certain HIV medicines and become harder to treat. You should get an HIV test before you start taking Baraclude and anytime after that when there is a chance you were exposed to HIV.
Baraclude can cause serious side effects including:
3. Lactic acidosis (buildup of acid in the blood). Some people who have taken Baraclude or medicines like Baraclude (a nucleoside analogue) have developed a serious condition called lactic acidosis. Lactic acidosis is a serious medical emergency that can cause death. Lactic acidosis must be treated in the hospital. Reports of lactic acidosis with Baraclude generally involved patients who were seriously ill due to their liver disease or other medical condition.
Call your healthcare provider right away if you get any of the following signs or symptoms of lactic acidosis:
- You feel very weak or tired.
- You have unusual (not normal) muscle pain.
- You have trouble breathing.
- You have stomach pain with nausea and vomiting.
- You feel cold, especially in your arms and legs.
- You feel dizzy or light-headed.
- You have a fast or irregular heartbeat.
4. Serious liver problems. Some people who have taken medicines like Baraclude have developed serious liver problems called hepatotoxicity, with liver enlargement (hepatomegaly) and fat in the liver (steatosis). Hepatomegaly with steatosis is a serious medical emergency that can cause death.
Call your healthcare provider right away if you get any of the following signs or symptoms of liver problems:
- Your skin or the white part of your eyes turns yellow (jaundice).
- Your urine turns dark.
- Your bowel movements (stools) turn light in color.
- You don’t feel like eating food for several days or longer.
- You feel sick to your stomach (nausea).
- You have lower stomach pain.
You may be more likely to get lactic acidosis or serious liver problems if you are female, very overweight, or have been taking nucleoside analogue medicines, like Baraclude, for a long time.
What should I tell my healthcare provider before taking Baraclude?
Before you take Baraclude, tell your healthcare provider if you:
- have kidney problems. Your Baraclude dose or schedule may need to be changed.
- have received medicine for HBV before. Some people, especially those who have already been treated with certain other medicines for HBV infection, may develop resistance to Baraclude. These people may have less benefit from treatment with Baraclude and may have worsening of hepatitis after resistant virus appears. Your healthcare provider will test the level of the hepatitis B virus in your blood regularly.
- have any other medical conditions.
- are pregnant or plan to become pregnant. It is not known if Baraclude will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
Antiretroviral Pregnancy Registry. If you take Baraclude while you are pregnant, talk to your healthcare provider about how you can take part in the Baraclude Antiretroviral Pregnancy Registry. The purpose of the pregnancy registry is to collect information about the health of you and your baby. - are breastfeeding or plan to breastfeed. It is not known if Baraclude can pass into your breast milk. You and your healthcare provider should decide if you will take Baraclude or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you have taken a medicine to treat HBV in the past.
Know the medicines you take. Keep a list of your medicines with you to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Baraclude?
- Take Baraclude exactly as your healthcare provider tells you to.
- Your healthcare provider will tell you how much Baraclude to take.
- Your healthcare provider will tell you when and how often to take Baraclude.
- Take Baraclude on an empty stomach, at least 2 hours after a meal and at least 2 hours before the next meal.
|
 |
|
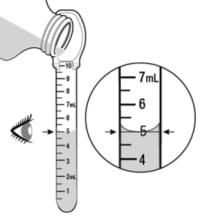 |
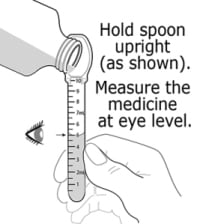
- Baraclude Oral Solution should be swallowed directly from the dosing spoon.
- Baraclude Oral Solution should not be mixed with water or any other liquid.
- After each use, rinse the dosing spoon with water and allow it to air dry.
- If you lose the dosing spoon, call your pharmacist or healthcare provider for instructions.
- Do not change your dose or stop taking Baraclude without talking to your healthcare provider.
- If you miss a dose of Baraclude, take it as soon as you remember and then take your next dose at its regular time. If it is almost time for your next dose, skip the missed dose. Do not take two doses at the same time. Call your healthcare provider or pharmacist if you are not sure what to do.
- When your supply of Baraclude starts to run low, call your healthcare provider or pharmacy for a refill. Do not run out of Baraclude.
- If you take too much Baraclude, call your healthcare provider or go to the nearest emergency room right away.
What are the possible side effects of Baraclude?
Baraclude may cause serious side effects. See “What is the most important information I should know about Baraclude?”
The most common side effects of Baraclude include:
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Baraclude. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
General information about the safe and effective use of Baraclude
Baraclude does not stop you from spreading the hepatitis B virus (HBV) to others by sex, sharing needles, or being exposed to your blood. Talk with your healthcare provider about safe sexual practices that protect your partner. Never share needles. Do not share personal items that can have blood or body fluids on them, like toothbrushes or razor blades. A shot (vaccine) is available to protect people at risk from becoming infected with HBV.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Baraclude for a condition for which it was not prescribed. Do not give Baraclude to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about Baraclude. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about Baraclude that is written for health professionals.
For more information, go to www.Baraclude.com or call 1-800-321-1335.
How should I store Baraclude?
- Store Baraclude Tablets or Oral Solution at room temperature, between 68°F and 77°F (20°C and 25°C).
- Keep Baraclude Tablets in a tightly closed container.
- Store Baraclude Tablets or Baraclude Oral Solution in the original carton, and keep the carton out of the light.
- Safely throw away Baraclude that is out of date or no longer needed. Dispose of unused medicines through community take-back disposal programs when available or place Baraclude in an unrecognizable closed container in the household trash.
Keep Baraclude and all medicines out of the reach of children.
What are the ingredients in Baraclude?
Active ingredient: entecavir
Inactive ingredients in Baraclude Tablets: lactose monohydrate, microcrystalline cellulose, crospovidone, povidone, magnesium stearate.
Tablet film-coat: titanium dioxide, hypromellose, polyethylene glycol 400, polysorbate 80 (0.5 mg tablet only), and iron oxide red (1 mg tablet only).
Inactive ingredients in Baraclude Oral Solution: maltitol, sodium citrate, citric acid, methylparaben, propylparaben, and orange flavor.
Label
BARACLUDE 0.5 MG TABLETS REPRESENTATIVE PACKAGING
30 Tablets NDC 0003-1611-12
Baraclude®
(entecavir)
Tablets
0.5 mg
Bristol-Myers Squibb
Rx only


BARACLUDE 1 MG TABLETS REPRESENTATIVE PACKAGING
30 Tablets NDC 0003-1612-12
Baraclude®
(entecavir)
Tablets
1 mg
Bristol-Myers Squibb
Rx only


