Zonegran
Generic name: zonisamide
Drug class: Carbonic anhydrase inhibitor anticonvulsants
Medically reviewed by A Ras MD.
What is Zonegran?
Zonegran is a prescription medicine that is used with other medicines to treat partial seizures in adults.
It is not known if Zonegran is safe or effective in children under 16 years of age.
Description
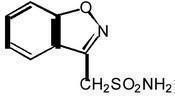
ZONEGRAN® (zonisamide) is an antiseizure drug chemically classified as a sulfonamide and unrelated to other antiseizure agents. The active ingredient is zonisamide, 1,2-benzisoxazole-3-methanesulfonamide. The empirical formula is C8H8N2O3S with a molecular weight of 212.23. Zonisamide is a white powder, pKa = 10.2, and is moderately soluble in water (0.80 mg/mL) and 0.1 N HCl (0.50 mg/mL).
The chemical structure is:
ZONEGRAN is supplied for oral administration as capsules containing 25 mg or 100 mg zonisamide.
Each 25 mg capsule contains the labeled amount of zonisamide plus the following inactive ingredients: microcrystalline cellulose, hydrogenated vegetable oil, sodium lauryl sulfate, gelatin, and titanium dioxide.
Each 100 mg capsule contains the labeled amount of zonisamide plus the following inactive ingredients: microcrystalline cellulose, hydrogenated vegetable oil, sodium lauryl sulfate, gelatin, titanium dioxide, FD&C Red No. 40 and FD&C Yellow No. 6.
Mechanism of Action:
The precise mechanism(s) by which zonisamide exerts its antiseizure effect is unknown. Zonisamide demonstrated anticonvulsant activity in several experimental models. In animals, zonisamide was effective against tonic extension seizures induced by maximal electroshock but ineffective against clonic seizures induced by subcutaneous pentylenetetrazol. Zonisamide raised the threshold for generalized seizures in the kindled rat model and reduced the duration of cortical focal seizures induced by electrical stimulation of the visual cortex in cats. Furthermore, zonisamide suppressed both interictal spikes and the secondarily generalized seizures produced by cortical application of tungstic acid gel in rats or by cortical freezing in cats. The relevance of these models to human epilepsy is unknown.
Zonisamide may produce these effects through action at sodium and calcium channels. In vitro pharmacological studies suggest that zonisamide blocks sodium channels and reduces voltage-dependent, transient inward currents (T-type Ca2+ currents), consequently stabilizing neuronal membranes and suppressing neuronal hypersynchronization. In vitro binding studies have demonstrated that zonisamide binds to the GABA/benzodiazepine receptor ionophore complex in an allosteric fashion which does not produce changes in chloride flux.
Other in vitro studies have demonstrated that zonisamide (10–30 µg/mL) suppresses synaptically-driven electrical activity without affecting postsynaptic GABA or glutamate responses (cultured mouse spinal cord neurons) or neuronal or glial uptake of [3H]-GABA (rat hippocampal slices). Thus, zonisamide does not appear to potentiate the synaptic activity of GABA. In vivo microdialysis studies demonstrated that zonisamide facilitates both dopaminergic and serotonergic neurotransmission.
Zonisamide is a carbonic anhydrase inhibitor. The contribution of this pharmacological action to the therapeutic effects of zonisamide is unknown. However, as a carbonic anhydrase inhibitor, zonisamide may cause metabolic acidosis
What is the most important information I should know about Zonegran?
Zonegran may cause serious side effects, including:
- Serious skin rash that can cause death.
- Serious allergic reactions that may affect different parts of the body.
- Less sweating and increase in your body temperature (fever).
- Suicidal thoughts or actions in some people.
- Increased level of acid in your blood (metabolic acidosis).
- Problems with your concentration, attention, memory, thinking, speech, or language.
- Blood cell changes such as reduced red and white blood cell counts.
These serious side effects are described below.
1. Zonegran may cause a serious skin rash that can cause death. These serious skin reactions are more likely to happen when you begin taking Zonegran within the first 4 months of treatment but may occur at later times.
2. Zonegran can cause other types of allergic reactions or serious problems that may affect different parts of the body such as your liver, kidneys, heart, or blood cells. You may or may not have a rash with these types of reactions. These reactions can be very serious and can cause death. Call your health care provider right away if you have:
- fever
- severe muscle pain
- rash
- swollen lymph glands
- swelling of your face
- unusual bruising or bleeding
- weakness, fatigue
- yellowing of your skin or the white part of your eyes
3. Zonegran may cause you to sweat less and to increase your body temperature (fever). You may need to be hospitalized for this. You should watch for decreased sweating and fever, especially when it is hot and especially in children taking Zonegran.
Call your health care provider right away if you have:
- high fever, recurring fever, or long lasting fever
- less sweat than normal
4. Like other antiepileptic drugs, Zonegran may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempt to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop Zonegran without first talking to a healthcare provider. Stopping Zonegran suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
5. Zonegran can increase the level of acid in your blood (metabolic acidosis). If left untreated, metabolic acidosis can cause brittle or soft bones (osteoporosis, osteomalacia, osteopenia), kidney stones and can slow the rate of growth in children. Metabolic acidosis can happen with or without symptoms.
Sometimes people with metabolic acidosis will:
- feel tired
- not feel hungry (loss of appetite)
- feel changes in heartbeat
- have trouble thinking clearly
Your healthcare provider should do a blood test to measure the level of acid in your blood before and during your treatment with Zonegran.
6. Zonegran may cause problems with your concentration, attention, memory, thinking, speech, or language.
7. Zonegran can cause blood cell changes such as reduced red and white blood cell counts. Call your healthcare provider if you develop fever, sore throat, sores in your mouth, or unusual bruising.
Zonegran can have other serious side effects. For more information ask your healthcare provider or pharmacist. Tell your healthcare provider if you have any side effect that bothers you. Be sure to read the section titled “What are the possible side effects of Zonegran?”
Who should not take Zonegran?
Do not take Zonegran if you are allergic to medicines that contain sulfa.
What should I tell my healthcare provider before taking Zonegran?
Before taking Zonegran, tell your healthcare provider about all your medical conditions, including if you:
- have or have had depression, mood problems or suicidal thoughts or behavior
- have kidney problems
- have liver problems
- have a history of metabolic acidosis (too much acid in your blood)
- have weak, brittle bones or soft bones (osteomalacia, osteopenia or osteoporosis)
- have a growth problem
- are on a diet high in fat called a ketogenic diet
- have diarrhea
Tell your healthcare provider if you:
- are pregnant or plan to become pregnant. Zonegran may harm your unborn baby. Women who can become pregnant should use effective birth control. Tell your healthcare provider right away if you become pregnant while taking Zonegran.
You and your healthcare provider should decide if you should take Zonegran while you are pregnant.
If you become pregnant while taking Zonegran, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy. - are breastfeeding or plan to breastfeed. Zonegran can pass into your breast milk. It is not known if Zonegran in your breast milk can harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take Zonegran.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
How should I take Zonegran?
- Take Zonegran exactly as prescribed. Your healthcare prescriber may change your dose. Your healthcare provider will tell you how much Zonegran to take.
- Take Zonegran with or without food.
- Swallow the capsules whole.
- If you take too much Zonegran, call your local Poison Control Center or go to the nearest emergency room right away.
- Do not stop taking Zonegran without talking to your healthcare provider. Stopping Zonegran suddenly can cause serious problems, including seizures that will not stop (status epilepticus).
What should I avoid while taking Zonegran?
- Do not drink alcohol or take other drugs that make you sleepy or dizzy while taking Zonegran until you talk to your health care provider. Zonegran taken with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how Zonegran affects you. Zonegran can slow your thinking and motor skills.
What are the possible side effects of Zonegran?
Zonegran can cause serious side effects. See “What is the most important information I should know about Zonegran?”
Other serious side effects include:
- kidney stones. Back pain, stomach pain, or blood in your urine may mean you have kidney stones. Drink plenty of fluids while you take Zonegran to lower your chance of getting kidney stones.
- problems with mood or thinking (new or worse depression; sudden changes in mood, behavior, or loss of contact with reality, sometimes associated with hearing voices or seeing things that are not really there; feeling sleepy or tired; trouble concentrating; speech and language problems). Call your healthcare provider right away if you have any of the symptoms listed above.
The most common side effects of Zonegran include:
- drowsiness
- loss of appetite
- dizziness
- problems with concentration or memory
- trouble with walking and coordination
- agitation or irritability
Side effects can happen at any time, but are more likely to happen during the first several weeks after starting Zonegran.
These are not all of the possible side effects of Zonegran. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Zonegran
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Zonegran for a condition for which it was not prescribed. Do not give Zonegran to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Zonegran that is written for health professionals.
How should I store Zonegran?
- Store Zonegran between 59°F to 86°F (15°C to 30°C)
- Keep Zonegran dry and away from light
Keep Zonegran and all medicines out of the reach of children.
What are the ingredients in Zonegran?
Active ingredient: zonisamide
Inactive ingredients:
25 mg capsules: microcrystalline cellulose, hydrogenated vegetable oil, sodium lauryl sulfate, gelatin, and titanium dioxide
100 mg capsules: microcrystalline cellulose, hydrogenated vegetable oil, sodium
Label
PRINCIPAL DISPLAY PANEL – 25 mg Capsules
- NDC 59212-681-10
- Zonegran®
(zonisamide) capsules
25 mg
100 capsules - Rx only
- Dispense accompanying Medication Guide to each patient.
- Mfd. for: Concordia Pharmaceuticals

SRC: NLM .
