Xtandi
Generic name: enzalutamide
Drug classes: Antiandrogens, Hormones / antineoplastics
Medically reviewed by A Ras MD.
What is Xtandi?
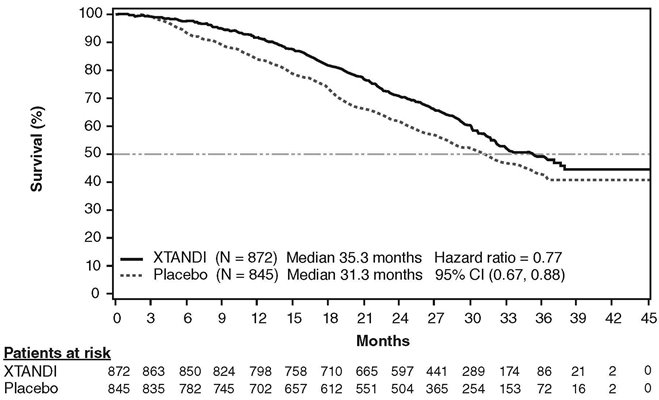
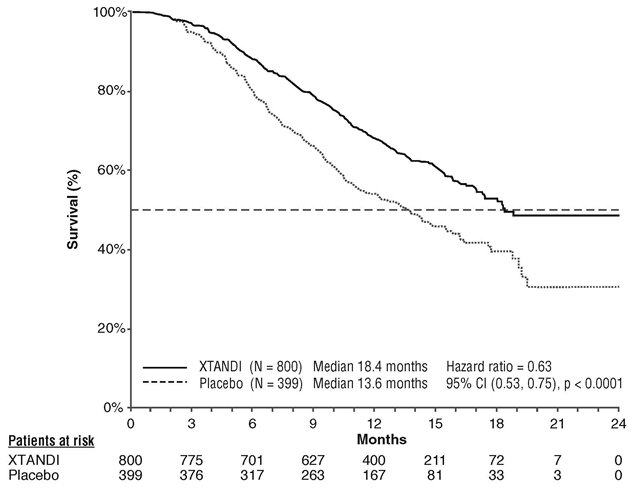
Xtandi is a prescription medicine used to treat men with prostate cancer that no longer responds to a hormone therapy or surgical treatment to lower testosterone OR has spread to other parts of the body and responds to a hormone therapy or surgical treatment to lower testosterone.
It is not known if Xtandi is safe and effective in females.
It is not known if Xtandi is safe and effective in children.
Description
Enzalutamide is an androgen receptor inhibitor. The chemical name is 4-{3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl}-2-fluoro-N-methylbenzamide.
The molecular weight is 464.44 and molecular formula is C21H16F4N4O2S. The structural formula is:
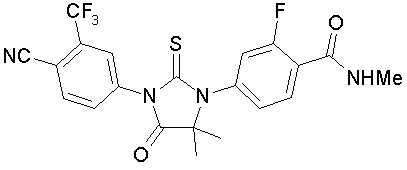
Enzalutamide is a white crystalline non-hygroscopic solid. It is practically insoluble in water.
XTANDI is available as liquid-filled soft gelatin capsules for oral administration. Each capsule contains 40 mg of enzalutamide as a solution in caprylocaproyl polyoxylglycerides. The inactive ingredients are caprylocaproyl polyoxylglycerides, butylated hydroxyanisole, butylated hydroxytoluene, gelatin, sorbitol sorbitan solution, glycerin, purified water, titanium dioxide, and black iron oxide.
XTANDI is also available as film-coated tablets for oral administration. Each tablet contains 40 mg or 80 mg of enzalutamide. The inactive ingredients are hypromellose acetate succinate, microcrystalline cellulose, colloidal silicon dioxide, croscarmellose sodium, and magnesium stearate. The tablet film-coat contains hypromellose, talc, polyethylene glycol, titanium dioxide, and ferric oxide.
Mechanism of Action
Enzalutamide is an androgen receptor inhibitor that acts on different steps in the androgen receptor signaling pathway. Enzalutamide has been shown to competitively inhibit androgen binding to androgen receptors; and consequently, inhibits nuclear translocation of androgen receptors and their interaction with DNA. A major metabolite, N‑desmethyl enzalutamide, exhibited similar in vitro activity to enzalutamide. Enzalutamide decreased proliferation and induced cell death of prostate cancer cells in vitro, and decreased tumor volume in a mouse prostate cancer xenograft model.
What should I tell my healthcare provider before taking Xtandi?
Before taking Xtandi, tell your healthcare provider about all your medical conditions, including if you:
- have a history of seizures, brain injury, stroke, or brain tumors.
- have a history of heart disease.
- have high blood pressure.
- have abnormal amounts of fat or cholesterol in your blood (dyslipidemia).
- are pregnant or plan to become pregnant. Xtandi can cause harm to your unborn baby and loss of pregnancy (miscarriage).
- have a partner who is pregnant or may become pregnant.
- Males who have female partners who are able to become pregnant should use effective birth control (contraception) during treatment with Xtandi and for 3 months after the last dose of Xtandi.
- Males must use a condom during sex with a pregnant female.
- are breastfeeding or plan to breastfeed. It is not known if Xtandi passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Xtandi may affect the way other medicines work, and other medicines may affect how Xtandi works.
You should not start or stop any medicine before you talk with the healthcare provider that prescribed Xtandi.
Know the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Xtandi?
- Take Xtandi exactly as your healthcare provider tells you.
- Take your prescribed dose of Xtandi 1 time a day, at the same time each day.
- Your healthcare provider may change your dose if needed.
- Do not change or stop taking your prescribed dose of Xtandi without talking with your healthcare provider first.
- Xtandi can be taken with or without food.
- Swallow Xtandi capsules or tablets whole. Do not chew, dissolve, or open the capsules. Do not cut, crush, or chew the tablets.
- If you are receiving gonadotropin-releasing hormone (GnRH) therapy, you should continue with this treatment during your treatment with Xtandi unless you have had a surgery to lower the amount of testosterone in your body (surgical castration).
- If you miss a dose of Xtandi, take your prescribed dose as soon as you remember that day. If you miss your daily dose, take your prescribed dose at your regular time the next day. Do not take more than your prescribed dose of Xtandi each day.
If you take too much Xtandi, call your healthcare provider or go to the nearest emergency room right away. You may have an increased risk of seizure if you take too much Xtandi.
What are the possible side effects of Xtandi?
Xtandi may cause serious side effects including:
- Seizure. If you take Xtandi you may be at risk of having a seizure. You should avoid activities where a sudden loss of consciousness could cause serious harm to yourself or others. Tell your healthcare provider right away if you have loss of consciousness or seizure.
- Posterior Reversible Encephalopathy Syndrome (PRES). If you take Xtandi you may be at risk of developing a condition involving the brain called PRES. Tell your healthcare provider right away if you have a seizure or quickly worsening symptoms such as headache, decreased alertness, confusion, reduced eyesight, blurred vision or other visual problems. Your healthcare provider will do a test to check for PRES.
- Allergic Reactions. Allergic reactions have happened in people who take Xtandi. Stop taking Xtandi and get medical help right away if you develop swelling of the face, tongue, lip or throat.
- Heart disease. Blockage of the arteries in the heart (ischemic heart disease) that can lead to death has happened in some people during treatment with Xtandi. Your healthcare provider will monitor you for signs and symptoms of heart problems during your treatment with Xtandi. Call your healthcare provider or go to the nearest emergency room right away if you get chest pain or discomfort at rest or with activity or shortness of breath during your treatment with Xtandi.
- Falls and fractures. Xtandi treatment may increase your risk for falls and fractures. Falls were not caused by loss of consciousness (fainting) or seizures. Your healthcare provider will monitor your risks for falls and fractures during treatment with Xtandi.
Your healthcare provider will stop treatment with Xtandi if you have serious side effects.
The most common side effects of Xtandi include:
- weakness or feeling more tired than usual
- back pain
- hot flashes
- constipation
- joint pain
- decreased appetite
- diarrhea
- high blood pressure
Xtandi may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of Xtandi. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Xtandi
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Xtandi for a condition for which it was not prescribed. Do not give Xtandi to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about Xtandi that is written for health professionals.
How should I store Xtandi?
- Store Xtandi between 68°F to 77°F (20°C to 25°C).
- Keep Xtandi capsules and tablets dry and in a tightly closed container.
Keep Xtandi and all medicines out of the reach of children.
What are the ingredients in Xtandi?
Active ingredient: enzalutamide
Inactive ingredients:
Capsules: caprylocaproyl polyoxylglycerides, butylated hydroxyanisole, butylated hydroxytoluene, gelatin, sorbitol sorbitan solution, glycerin, purified water, titanium dioxide, black iron oxide
Tablets: hypromellose acetate succinate, microcrystalline cellulose, colloidal silicon dioxide, croscarmellose sodium, and magnesium stearate.
The tablet film-coat contains hypromellose, talc, polyethylene glycol, titanium dioxide, and ferric oxide.
Label
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 0469-0625-99
- Rx Only
- Xtandi®
(enzalutamide)
tablets - 40 mg
- Swallow tablets whole.
Do not cut, crush, or chew the tablets. - 120 Tablets
- Manufactured by Pfizer Pharmaceuticals LLC, for Astellas Pharma US, Inc., Northbrook, IL 60062
- PAA135442
- 234833-XTA-USA
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- NDC 0469-0725-60
- Rx Only
- Xtandi®
(enzalutamide)
tablets - 80 mg
- Swallow tablets whole.
Do not cut, crush, or chew the tablets. - 60 Tablets
- Manufactured by Pfizer Pharmaceuticals LLC, for Astellas Pharma US, Inc., Northbrook, IL 60062
- PAA135444
- 234833-XTA-USA