Xcopri
Generic name: cenobamate
Dosage form: Tablets
Drug class: Carbamate anticonvulsants
Medically reviewed by A Ras MD.
What is Xcopri?
Xcopri is a prescription medicine used to treat partial-onset seizures in adults.
It is not known if Xcopri is safe and effective in children.
Description
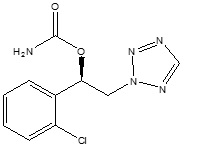
The chemical name of XCOPRI (cenobamate) is [(1R)-1-(2-Chlorophenyl)-2-(tetrazol-2-yl) ethyl] carbamate. Its molecular formula is C10H10ClN5O2 and its molecular weight is 267.67 g/mol. The chemical structure is:
XCOPRI is a white to off-white crystalline powder. It is very soluble in aqueous solutions (water 1.7 mg/mL) and has higher solubility in organic solvents like ethanol (209.4 mg/mL).
XCOPRI tablets are for oral administration and contain the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate and film coating agents specified below:
12.5 mg tablets: Not applicable, since 12.5 mg tablets are uncoated.
25 mg and 100 mg tablets: FD&C Blue# 2/indigo carmine aluminum lake, iron oxide red, iron oxide yellow, polyethylene glycol 3350, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide.
50 mg tablets: iron oxide yellow, polyethylene glycol 3350, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide.
150 mg and 200 mg tablets: iron oxide red, iron oxide yellow, polyethylene glycol 3350, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide.
Mechanism of Action
The precise mechanism by which cenobamate exerts its therapeutic effects in patients with partial-onset seizures is unknown. Cenobamate has been demonstrated to reduce repetitive neuronal firing by inhibiting voltage-gated sodium currents. It is also a positive allosteric modulator of the γ-aminobutyric acid (GABAA) ion channel.
What is the most important information I should know about Xcopri?
Xcopri can cause serious side effects, including:
Serious or life threatening allergic reactions which may affect organs and other parts of your body like the liver or blood cells. You may or may not have a rash with these types of reactions.
Call your healthcare provider right away and go to the nearest emergency room if you have any of the following:
- swelling of your face, eyes, lips, or tongue
- trouble swallowing or breathing
- a skin rash
- hives
- fever, swollen glands, or sore throat that does not go away or comes and goes
- painful sores in the mouth or around your eyes
- yellowing of your skin or eyes
- unusual bruising or bleeding
- severe fatigue or weakness
- severe muscle pain
- frequent infections that do not go away
These symptoms may be the first signs of a serious reaction. A healthcare provider should examine you to decide if you should continue taking Xcopri.
Like other antiepileptic drugs, Xcopri may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
- Suicidal thoughts or actions may be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop taking Xcopri without first talking to your healthcare provider.
- Stopping Xcopri suddenly can cause serious problems.
- Stopping a seizure medicine suddenly can cause seizures that will not stop (status epilepticus).
- Xcopri is a federally controlled substance (CV) because it can be abused or lead to dependence. Keep Xcopri in a safe place to prevent misuse and abuse. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines, or street drugs. Selling or giving away Xcopri may harm others and is against the law.
Who should not take Xcopri?
Do not take Xcopri if you:
- are allergic to cenobamate, any of the other ingredients in Xcopri. See the end of this Medication Guide for a complete list of ingredients in Xcopri.
- have a genetic problem (called familial short QT syndrome) that affects the electrical system of the heart.
What should I tell my healthcare provider before taking Xcopri?
Before taking Xcopri, tell your healthcare provider about all your medical conditions, including if you:
- have or had depression, mood problems, or suicidal thoughts or actions.
- have liver, kidney, or blood problems.
- have had an allergic reaction to a medicine which caused a rash or affected internal organs, like the liver or blood cells.
- use birth control medicine. Xcopri may cause your birth control medicine to be less effective. Talk to your healthcare provider about the best birth control method to use while taking Xcopri.
- are pregnant or plan to become pregnant. It is not known if Xcopri will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking Xcopri. You and your healthcare provider will decide if you should take Xcopri while you are pregnant.
- If you become pregnant while taking Xcopri, talk to your healthcare provider about registering with the North American Antiepileptic Drug (NAAED) Pregnancy Registry. The purpose of this registry is to collect information about the safety of antiepileptic medicine during pregnancy. You can enroll in this registry by calling 1-888-233-2334 or go to www.aedpregnancyregistry.org
- are breastfeeding or plan to breastfeed. It is not known if Xcopri passes into breastmilk. Talk to your healthcare provider about the best way to feed your baby while taking Xcopri.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Xcopri may affect the way other medicines work, and other medicines may affect how Xcopri works. Do not start a new medicine without first talking with your healthcare provider.
Ask your healthcare provider or pharmacist for a list of medicines you are taking, if you are not sure. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take Xcopri?
- Take Xcopri exactly as your healthcare provider tells you to take it. It is very important to increase your dose of Xcopri slowly, as instructed by your healthcare provider.
- Do not stop taking Xcopri without talking to your healthcare provider. Stopping Xcopri suddenly can cause serious problems, including seizures that will not stop (status epilepticus).
- Your healthcare provider may change your dose, if needed. Your healthcare provider will tell you how much Xcopri to take.
- Xcopri can be taken at any time of day, with or without food.
- Swallow tablets whole with liquid. Do not crush or chew.
- Talk with your healthcare provider about what you should do if you miss a dose.
- If you take too much Xcopri, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking Xcopri?
- Do not drive, operate machinery, or do other dangerous activities until you know how Xcopri affects you. Xcopri may slow your thinking and motor skills and may affect your vision.
- Do not drink alcohol or take other medicines that can make you sleepy or dizzy while taking Xcopri without first talking to your healthcare provider.
What are the possible side effects of Xcopri?
Xcopri may cause serious side effects, including:
- See “What is the most important information I should know about Xcopri?”
- problems with the electrical system of the heart (QT shortening). Call your healthcare provider if you have symptoms of QT shortening including fast heartbeat (heart palpitations) that last a long time or fainting.
- nervous system problems. Xcopri may cause problems that can affect your nervous system. Symptoms of nervous system problems include:
- dizziness
- trouble walking or with coordination
- feeling sleepy and tired
- trouble concentrating, remembering, and thinking clearly
- vision problems
The most common side effects of Xcopri include:
- feeling sleepy and tired
- dizziness
- double vision
- headache
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of Xcopri.
For more information, ask your healthcare provider or pharmacist.Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Xcopri
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Xcopri for a condition for which it was not prescribed. Do not give Xcopri to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Xcopri that is written for health professionals.
How should I store Xcopri?
- Store Xcopri at room temperature between 68℉ to 77℉ (20℃ to 25℃).
- Safely throw away medicine that is out of date or no longer needed.
- Keep Xcopri and all medicines out of the reach of children.
What are the ingredients in Xcopri?
Active ingredient: cenobamate
Inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
25 mg and 100 mg tablets: FD&C Blue #2/indigo carmine aluminum lake, iron oxide red, iron oxide yellow, polyethylene glycol 3350, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide.
50 mg tablets: iron oxide yellow, polyethylene glycol 3350, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide.
150 mg and 200 mg tablets: iron oxide red, iron oxide yellow, polyethylene glycol 3350, polyvinyl alcohol-part hydrolyzed, talc, and titanium dioxide.
Label
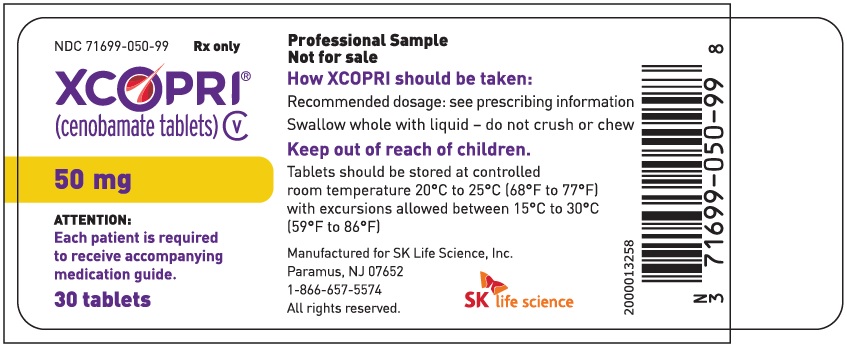
PRINCIPAL DISPLAY PANEL – NDC: 71699-050-99 – 50 MG 30-COUNT BOTTLE LABEL
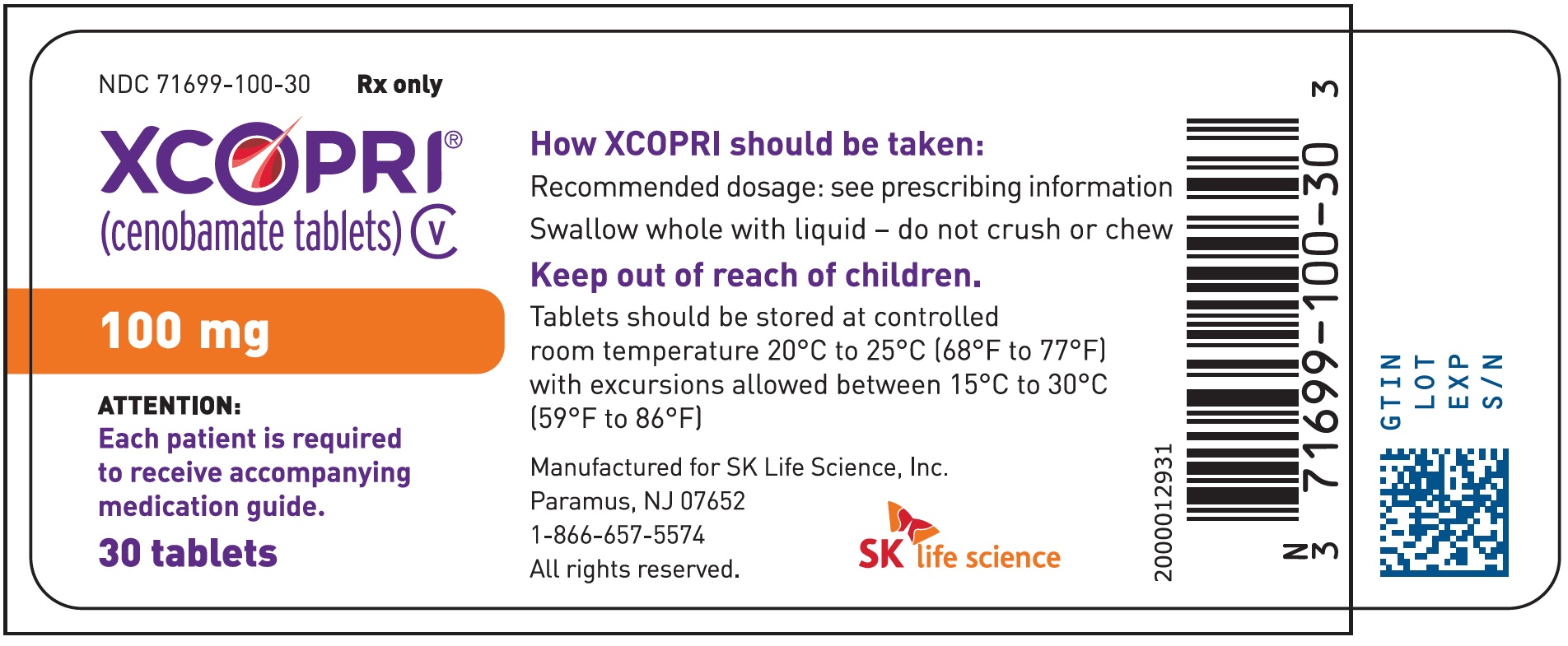
PRINCIPAL DISPLAY PANEL – NDC: 71699-100-30 – 100 MG 30-COUNT BOTTLE LABEL
PRINCIPAL DISPLAY PANEL – NDC: 71699-150-30 – 150 MG 30-COUNT BOTTLE LABEL

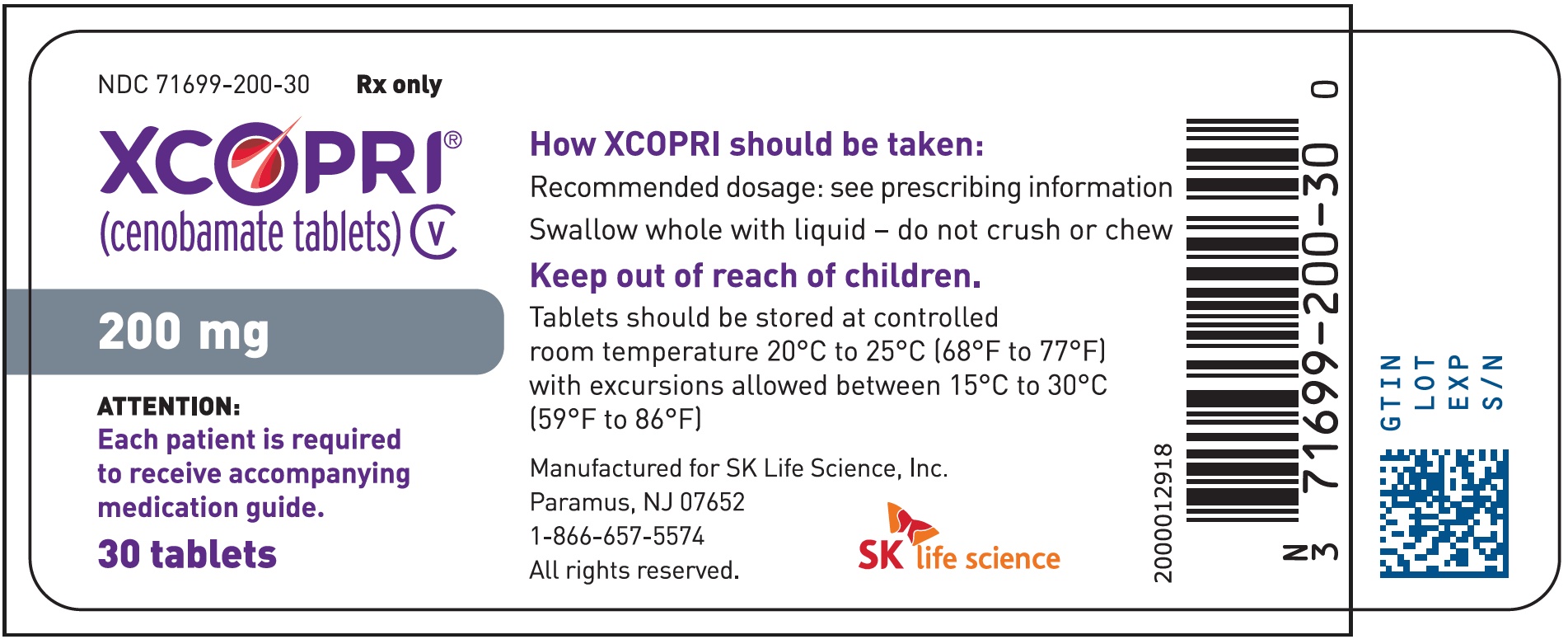
PRINCIPAL DISPLAY PANEL – NDC: 71699-200-30 – 200 MG 30-COUNT BOTTLE LABEL
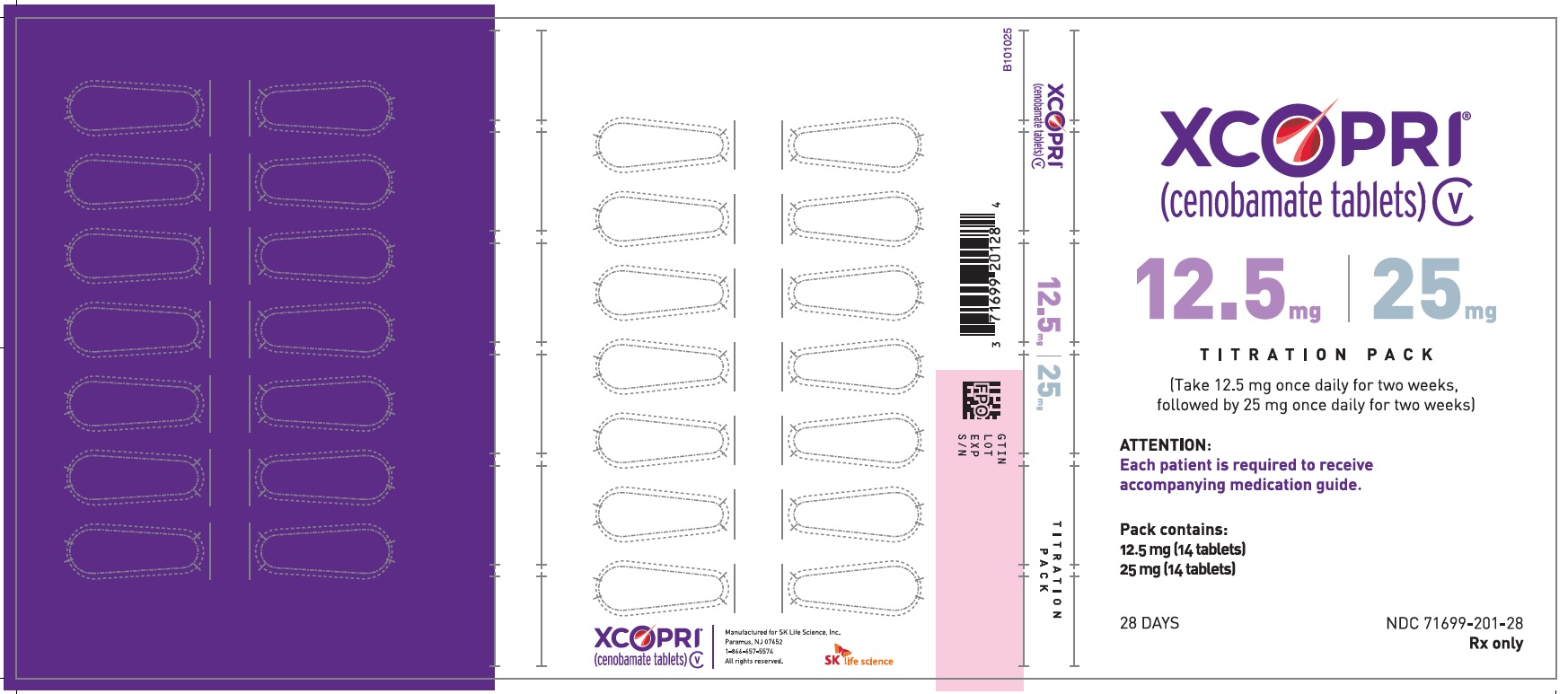
PRINCIPAL DISPLAY PANEL – NDC: 71699-201-28 – 12.5 MG 14-COUNT AND 25 MG 14-COUNT TITRATION PACK LABEL (FRONT)