Wynzora
Generic name: betamethasone and calcipotriene topical
Brand names: Enstilar, Taclonex, Wynzora
Drug class: Topical antipsoriatics
Medically reviewed by A Ras MD.
What is Wynzora?
Wynzora Cream is a prescription medicine used on the skin (topical) to treat plaque psoriasis in people 18 years of age and older.
It is not known if Wynzora Cream is safe and effective in children under 18 years of age
Description
WYNZORA (calcipotriene and betamethasone dipropionate) Cream contains anhydrous calcipotriene and betamethasone dipropionate intended for topical use.
Calcipotriene is a synthetic vitamin D3 analog.
Chemically, calcipotriene is (5Z,7E,22E,24S)-24-Cyclopropyl-9,10-secochola-5,7,10(19),22 tetraene-1α,3β,24-triol, with the empirical formula C27H40O3, a molecular weight of 412.6, and the following structural formula:
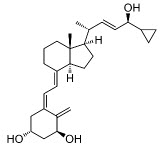
Calcipotriene is a white or almost white powder. It is insoluble in water, freely soluble in ethanol and slightly soluble in methylene chloride.
Betamethasone dipropionate is a synthetic corticosteroid.
Betamethasone dipropionate has the chemical name Pregna-1,4-diene-3,20-dione,9-fluoro-11-hydroxy-16-methyl-17,21-bis(1-oxypropoxy)-,(11β,16β), with the empirical formula C28H37FO7, a molecular weight of 504.6, and the following structural formula:
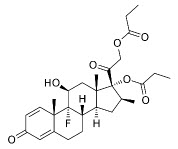
Betamethasone dipropionate is a white to almost white crystalline powder. It is practically insoluble in water, freely soluble in acetone and in methylene chloride, sparingly soluble in alcohol.
Each gram of WYNZORA Cream contains 50 mcg of calcipotriene and 0.644 mg of betamethasone dipropionate (equivalent to 0.5 mg of betamethasone). WYNZORA Cream also contains the following inactive ingredients: isopropyl myristate, mineral oil, medium-chain triglycerides, isopropyl alcohol, polyoxyl lauryl ether, poloxamer (407), polyoxyl 40 hydrogenated castor oil, carbomer interpolymer (type A), butylated hydroxyanisole, trolamine, dibasic sodium phosphate, heptahydrate, monobasic sodium phosphate, monohydrate, alpha-tocopherol and purified water.
Mechanism of Action
WYNZORA Cream combines the pharmacological effects of calcipotriene as a synthetic vitamin D3 analog and betamethasone dipropionate as a synthetic corticosteroid. However, while their pharmacologic and clinical effects are known, the exact mechanisms of their actions in plaque psoriasis are unknown.
What is the most important information I should know about Wynzora?
Wynzora Cream is for use on the skin only (topical use only). Do not get Wynzora Cream near or in your mouth, eyes, or vagina.
There are other medicines that contain the same medicine that is in Wynzora Cream and are used to treat plaque psoriasis. Do not use other products containing calcipotriene or a corticosteroid medicine with Wynzora Cream without talking to your healthcare provider first.
What should I tell my healthcare provider before using Wynzora?
Before using Wynzora Cream, tell your healthcare provider about all of your medical conditions, including if you:
- have a calcium metabolism disorder.
- have thinning skin (atrophy) at the treatment site.
- are pregnant or plan to become pregnant. It is not known if Wynzora Cream will harm your unborn baby. Wynzora Cream may increase your chance of having a low birth weight baby. If you use Wynzora Cream during pregnancy, use Wynzora Cream on the smallest area of the skin and for the shortest time needed.
- are breastfeeding or plan to breastfeed. It is not known if Wynzora Cream passes into your breast milk. Breastfeeding women should use Wynzora Cream on the smallest area of the skin and for the shortest time needed. Do not apply Wynzora Cream directly to the nipple and areola to avoid contact with your baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
How should I use Wynzora?
- Use Wynzora Cream exactly as prescribed by your healthcare provider.
- Your healthcare provider should tell you how much Wynzora Cream to use and where to use it.
- Apply Wynzora Cream to affected areas 1 time a day for up to 8 weeks. You should stop treatment when your plaque psoriasis is under control unless your healthcare provider gives you other instructions.
- You should not use more than 100 grams of Wynzora Cream in 1 week.
- Do not use Wynzora Cream longer than prescribed. Using too much Wynzora Cream, or using it too often or for too long, can increase your risk for having serious side effects.
- Do not use Wynzora Cream in the mouth, eyes, or vagina.
- Do not use Wynzora Cream on your face, groin, or armpits, or if you have thinning of your skin (atrophy) at the treatment site.
- If you accidentally get Wynzora Cream on your face or in your eyes wash the area with water right away.
- Wash your hands well after applying Wynzora Cream.
- Do not bandage or cover the treated skin area, unless instructed by your healthcare provider.
Applying Wynzora Cream:
- Remove the cap and check that the aluminum seal covers the opening on the top of the tube before the first use. To break the seal, turn the cap over and poke a hole through the seal.
- Gently rub Wynzora Cream all the way in to make sure that the plaques are well covered with the cream.
What are the possible side effects of Wynzora?
Wynzora Cream may cause serious side effects, including:
- Too much calcium in your blood or urine. Your healthcare provider may tell you to stop or temporarily stop treatment with Wynzora Cream if you have too much calcium in your blood or urine.
- Wynzora Cream can pass through your skin. Too much Wynzora Cream passing through your skin can cause your adrenal glands to stop working properly. Your healthcare provider may do blood tests to check for adrenal gland problems. Your healthcare provider may tell you to stop or temporarily stop treatment with Wynzora Cream.
- Cushing’s syndrome, a condition that happens when your body is exposed to large amounts of the hormone cortisol.
- High blood sugar (hyperglycemia).
- Skin problems. Tell your healthcare provider if you have any skin problems, including:
- thinning of your skin
- dryness
- burning
- changes in skin color
- inflammation
- redness
- itching
- infection
- irritation
- raised bumps on your skin
- Eye problems. Using Wynzora Cream may increase your chance of getting cataracts and glaucoma. Do not get Wynzora Cream in your eyes because it may cause eye irritation. Tell your healthcare provider if you have blurred vision or other vision problems during treatment with Wynzora Cream.
The most common side effects of Wynzora Cream include upper respiratory infection, headache and irritation at the treatment site.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1- 800-FDA-1088
General information about the safe and effective use of Wynzora
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information guide. Do not use Wynzora Cream for a condition for which it was not prescribed. Do not give Wynzora Cream to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Wynzora Cream that is written for health professionals.
How should I store Wynzora?
- Store Wynzora Cream at room temperature between 68°F to 77°F (20°C to 25°C) with the cap on the tube tightly closed.
- Do not freeze and protect Wynzora Cream from light and excessive heat.
- Keep Wynzora Cream out of the light.
- Throw away (discard) unused Wynzora Cream 6 months after it has been opened.
Keep Wynzora Cream and all medicines out of the reach of children.
What are the ingredients in Wynzora?
Active ingredients: calcipotriene and betamethasone dipropionate
Inactive ingredients: isopropyl myristate, mineral oil, medium-chain triglycerides, isopropyl alcohol, polyoxyl lauryl ether, poloxamer (407), polyoxyl 40 hydrogenated castor oil, carbomer interpolymer (type A), butylated hydroxyanisole, trolamine, dibasic sodium phosphate, heptahydrate, monobasic sodium phosphate, monohydrate, alpha-tocopherol and purified water.
Label
PRINCIPAL DISPLAY PANEL – 60 G TUBE CARTON
- Wynzora®
(calcipotriene and betamethasone dipropionate)
Cream, 0.005%/0.064% - NDC 73499-001-01
- Rx only
Net Wt. 60 g - For Topical Use Only
Not for ophthalmic, oral or intravaginal use

PRINCIPAL DISPLAY PANEL – 5 G TUBE CARTON
- NDC 73499-001-03
- Wynzora®
(calcipotriene and betamethasone dipropionate)
Cream, 0.005%/0.064% - Net Wt. 10 x 5 g
- Samples – Not for sale
- For Topical Use Only
Not for ophthalmic, oral or intravaginal use

SRC: NLM .
