Uptravi
Generic name: selexipag (oral/injection)
Drug class: Agents for pulmonary hypertension
Medically reviewed by A Ras MD.
What is Uptravi used for?
Uptravi is a prescription medicine that is used to treat high blood pressure in the lungs.
Description
UPTRAVI contains selexipag, a prostacyclin receptor agonist. The chemical name of selexipag is 2-{4-[(5,6-diphenylpyrazin-2-yl)(isopropyl)amino]butoxy}- N-(methylsulfonyl) acetamide. It has a molecular formula of C 26H 32N 4O 4S and a molecular weight of 496.62. Selexipag has the following structural formula:

Selexipag is a pale yellow crystalline powder that is practically insoluble in water. In the solid state selexipag is very stable, is not hygroscopic, and is not light sensitive.
UPTRAVI ® (selexipag) tablets: depending on the dose strength, each round film-coated tablet for oral administration contains 200, 400, 600, 800, 1000, 1200, 1400, or 1600 mcg of selexipag. The tablets include the following inactive ingredients: corn starch, D-mannitol, hydroxypropyl cellulose, low substituted hydroxypropyl cellulose, and magnesium stearate. The tablets are film coated with a coating material containing carnauba wax, hypromellose, propylene glycol, titanium dioxide, along with mixtures of iron oxide black, iron oxide red or iron oxide yellow.
UPTRAVI ® (selexipag) for injection: contains 1800 mcg of selexipag per vial. UPTRAVI for injection includes the following inactive ingredients: glycine (180 mg), phosphoric acid (3.53 mg), polysorbate 20 (10.8 mg) and sodium hydroxide (for pH adjustment). UPTRAVI for injection is provided in 10 mL Type I clear glass vials closed by a stopper and tear-off aluminum seal.
Mechanism of Action
Selexipag is a prostacyclin receptor (IP receptor) agonist that is structurally distinct from prostacyclin. Selexipag is hydrolyzed by carboxylesterase 1 to yield its active metabolite, which is approximately 37-fold as potent as selexipag. Selexipag and the active metabolite are selective for the IP receptor versus other prostanoid receptors (EP 1–4, DP, FP, and TP).
Before taking Uptravi, tell your doctor:
- If you are allergic to Uptravi; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have liver disease.
- If you are taking gemfibrozil.
- If you are breast-feeding or plan to breast-feed.
This is not a list of all drugs or health problems that interact with this medicine.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Uptravi with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Uptravi?
- Tell all of your health care providers that you take Uptravi. This includes your doctors, nurses, pharmacists, and dentists.
- Tell your doctor if you are pregnant or plan on getting pregnant. You will need to talk about the benefits and risks of using Uptravi while you are pregnant.
How is Uptravi best taken?
Use Uptravi as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- Take with or without food. Taking Uptravi with food may help with some side effects.
- Swallow whole. Do not chew, break, or crush.
- Keep taking Uptravi as you have been told by your doctor or other health care provider, even if you feel well.
What do I do if I miss a dose?
- Take a missed dose as soon as you think about it.
- If it is less than 6 hours until the next dose, skip the missed dose and go back to the normal time.
- Do not take 2 doses at the same time or extra doses.
- If you miss 3 days of Uptravi, call your doctor to find out what to do.
What are the side effects of Uptravi that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Trouble breathing that is new or worse.
- Wheezing or coughing.
- Feeling very tired or weak.
What are some other side effects of Uptravi?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Headache.
- Diarrhea.
- Jaw pain.
- Upset stomach or throwing up.
- Muscle or joint pain.
- Pain in arms or legs.
- Flushing.
- Not hungry.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out Uptravi?
- Store at room temperature.
- Store in a dry place. Do not store in a bathroom.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
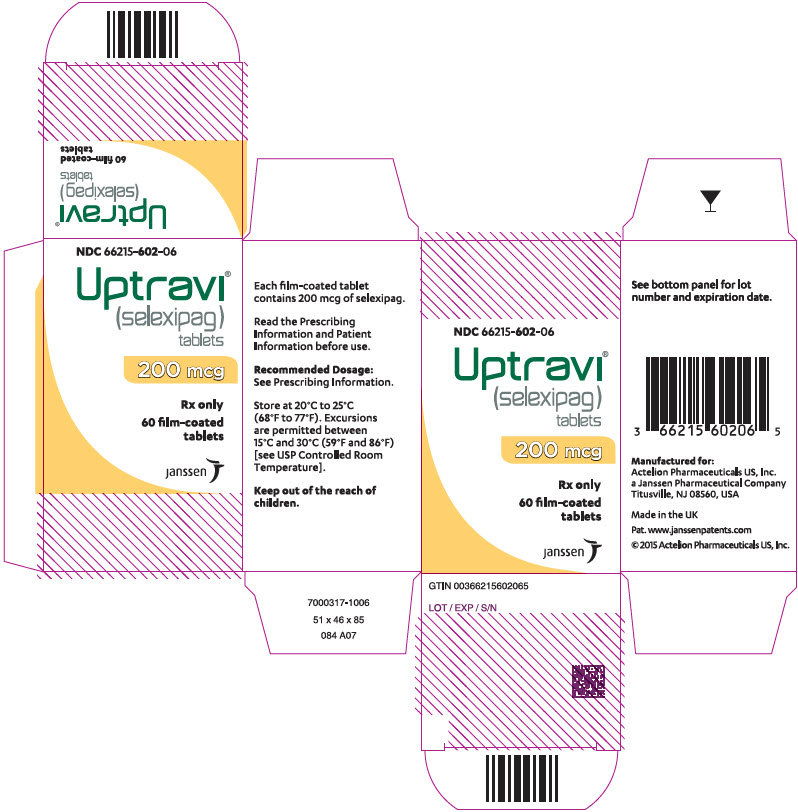
PRINCIPAL DISPLAY PANEL – 200 MCG TABLET BOTTLE CARTON
- NDC 66215-602-06
- Uptravi®
(selexipag)
tablets - 200 mcg
- Rx only
- 60 film-coated
tablets - janssen
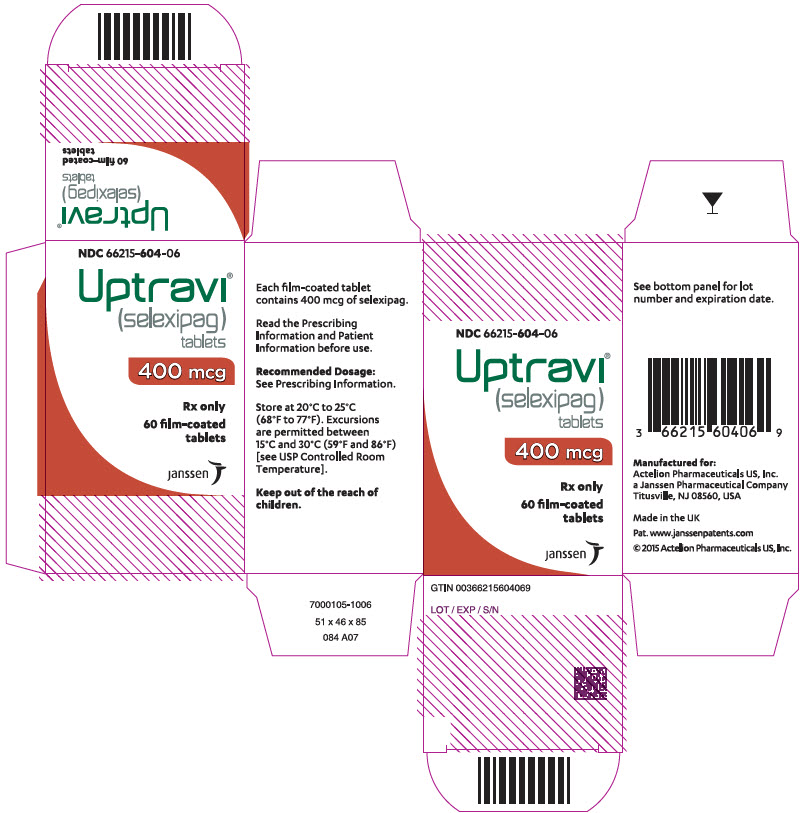
PRINCIPAL DISPLAY PANEL – 400 MCG TABLET BOTTLE CARTON
- NDC 66215-604-06
- Uptravi®
(selexipag)
tablets - 400 mcg
- Rx only
- 60 film-coated
tablets - janssen
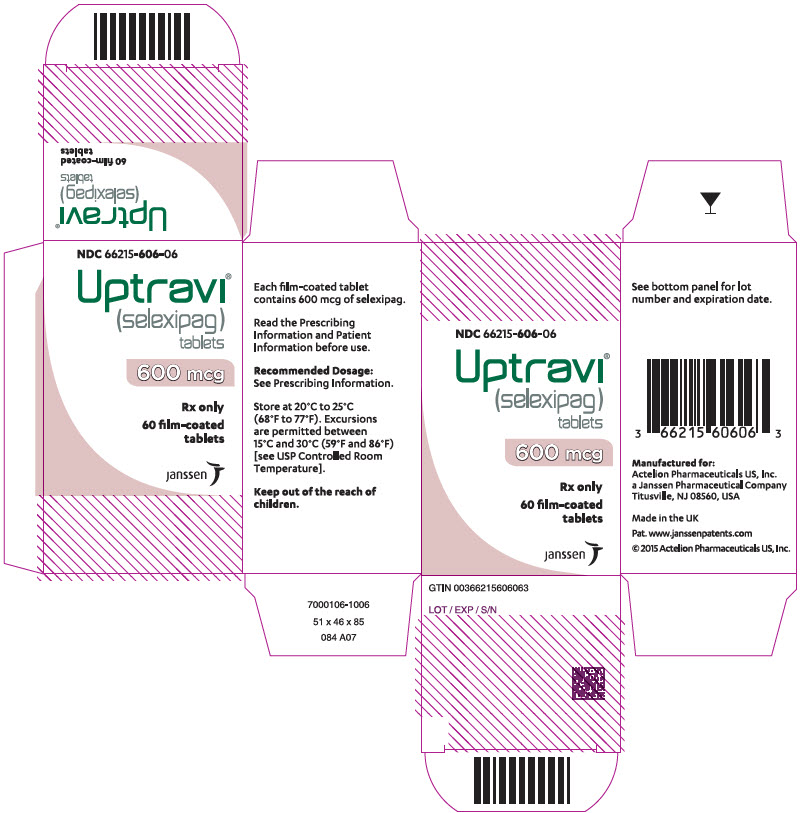
PRINCIPAL DISPLAY PANEL – 600 MCG TABLET BOTTLE CARTON
- NDC 66215-606-06
- Uptravi®
(selexipag)
tablets - 600 mcg
- Rx only
- 60 film-coated
tablets - janssen
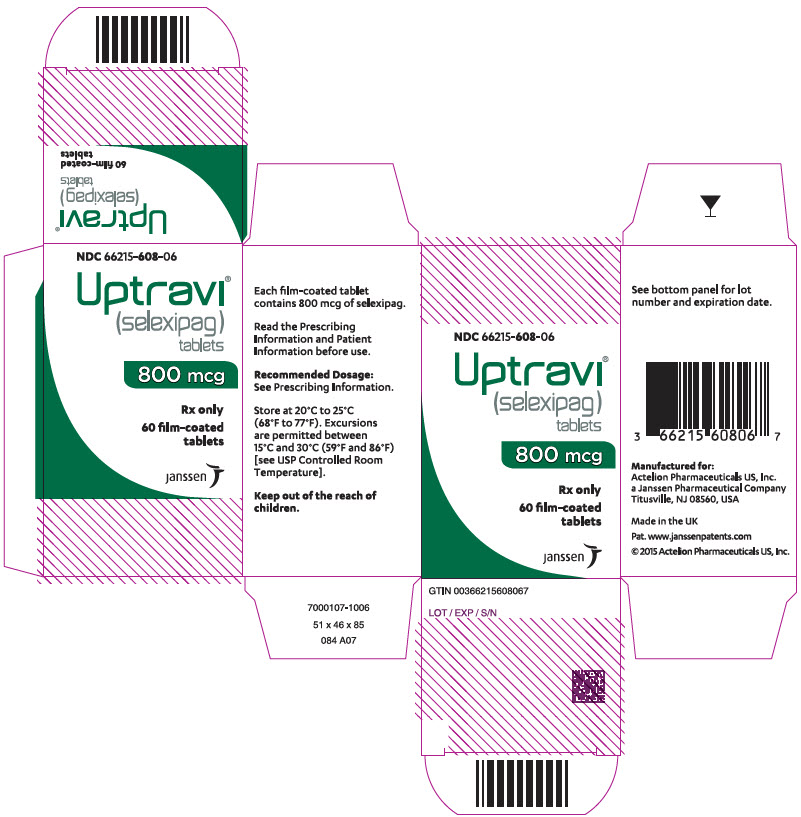
PRINCIPAL DISPLAY PANEL – 800 MCG TABLET BOTTLE CARTON
- NDC 66215-608-06
- Uptravi®
(selexipag)
tablets - 800 mcg
- Rx only
- 60 film-coated
tablets - janssen
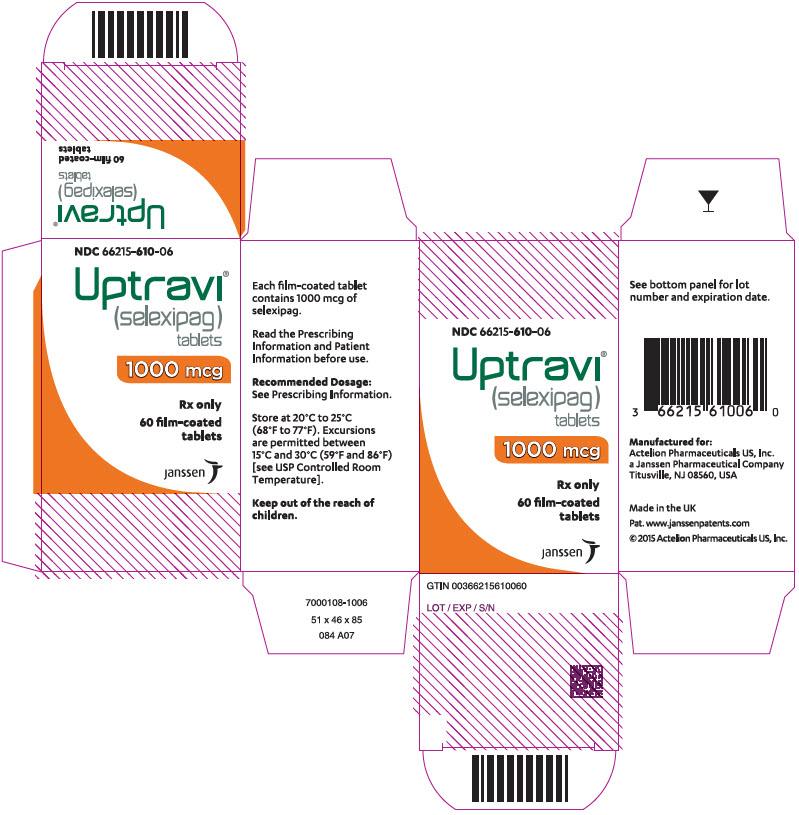
PRINCIPAL DISPLAY PANEL – 1000 MCG TABLET BOTTLE CARTON
- NDC 66215-610-06
- Uptravi®
(selexipag)
tablets - 1000 mcg
- Rx only
- 60 film-coated
tablets - janssen
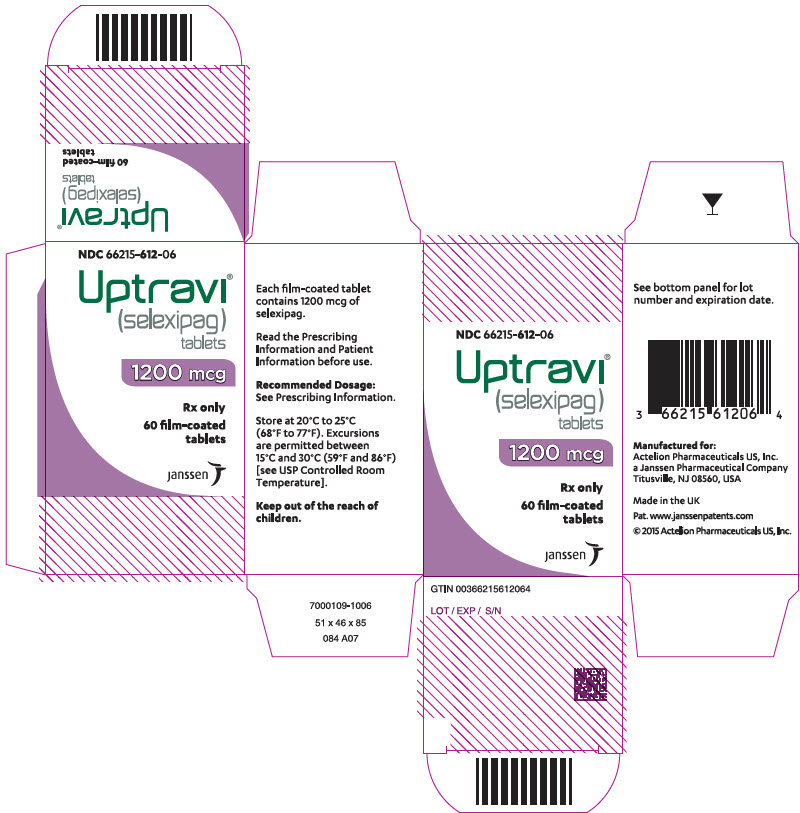
PRINCIPAL DISPLAY PANEL – 1200 MCG TABLET BOTTLE CARTON
- NDC 66215-612-06
- Uptravi®
(selexipag)
tablets - 1200 mcg
- Rx only
- 60 film-coated
tablets - janssen
SRC: NLM .
