Tocilizumab
Generic name: Tocilizumab
The brand name is Actemra
Drug class: Interleukin inhibitors
What is Tocilizumab?
 tocilizumab helps to reduce the effects of a substance within the body that may cause inflammation.
tocilizumab helps to reduce the effects of a substance within the body that may cause inflammation.
Tocilizumab is a medication used by the adult population to address:
- Moderate to extreme Rheumatoid arthritis that is moderate to severe following at least one other treatment was tried but did not be effective;
- large cells arteritis, (inflammation in the blood vessels’ lining which carries the blood of your heart into other areas within your body) and
- to reverse the decline in lung function that is caused by scleroderma to slow the decline in lung function caused by interstitial lung disease.
Tocilizumab can be used by the adult population and in children aged 2 and over to treat:
- multiarticular, or systemic juvenile joint (“Still disease”) as well
- severe Cytokine Release Syndrome (CRS is an immune system that is overactive to certain blood cell therapies used to fight cancer).
Tocilizumab can be prescribed with other medicines as part of a complete treatment.
Tocilizumab is also understudy to treat coronavirus. It is reported that the US Food and Drug Administration (FDA) has approved the emergency treatment with Tocilizumab together with steroid medication for treating children and adults aged 2 or more in hospital with COVID-19 and are receiving oxygen supplemental as well as a ventilator as well as ECMO (a cardiac machine which boosts oxygen levels in the blood).¶
Tocilizumab is not authorized to treat coronavirus, or COVID-19. This medication is not intended to be used in the treatment of COVID-19 in a non-hospital setting.
Warnings and precautions
Tocilizumab impacts the immune system. It is possible to contract infections more frequently, and even severe or fatal infections. Call your doctor in case you are suffering from fever or chills, pains, fatigue, cough wounds on your skin, diarrhea, weight loss, or burning sensations whenever you have to urinate.
Tocilizumab can also lead to perforations (a tear or hole) in your stomach or in the intestines. Consult your physician if you are experiencing stomach pain, or if you notice changes in your eating habits.
Tocilizumab can cause liver issues. Inform your doctor immediately in the event that you are experiencing right-sided stomach pains, vomiting, lack of appetite, fatigue and dark urine, stools that are clay-colored, or the appearance of yellowing on your eyes or skin.
Before starting treatment with Tocilizumab Your doctor will run tests to confirm that you don’t have tuberculosis or any other diseases. When you are taking Tocilizumab, it is possible that you may require regular medical tests.
in summary:
- For serious infections, do not apply Tocilizumab in the course of any active infections, even localized infections. If an infection becomes serious discontinue Tocilizumab to ensure that the infection has been controlled.
- Gastrointestinal (GI) perforation–use at a moderate rate for patients with a high risk.
- Hepatotoxicity- Check patients for symptoms and signs of hepatic injury. Change or stop using Tocilizumab when abnormal liver tests persist or get worse or when symptoms and signs of liver disease begin to manifest.
- Laboratory monitoring–recommended due to potential consequences of treatment-related changes in neutrophils, platelets, lipids, and liver function tests.
- Hypersensitivity reactions, such as anaphylaxis, and even death have occurred.
- Live vaccines–Do not use in conjunction with Tocilizumab.
Before taking this medication, you must consult your physician.
It is not recommended to use Tocilizumab in case you have an allergy to Tocilizumab.
Consult your physician when you notice any symptoms of infection like fever chills, cough fatigue, body aches, open skin wounds or sores and stomach pain or weight loss, painful urine, or the coughing up of blood.
Consult your physician whether you’ve had exposure to tuberculosis or recently went on a trip. Certain infections are more prevalent in specific regions of the world. Additionally, you might have come across the disease during your travels.
To be sure Tocilizumab is appropriate for you, ask your doctor if:
- an ongoing or chronic infection;
- Liver disease;
- diverticulitis, ulcers within your stomach or in your intestines,
- a condition that affects the nerves, like MS;
- diabetes;
- HIV (or an insufficient immune system)
- Hepatitis B (or if you’re carriers of the virus);
- cancer or
- If you’ve received or you are due to get any vaccinations.
The use of Tocilizumab could increase your chances of getting certain kinds of cancer. Consult your physician about this possibility.
Inform your doctor if are nursing or pregnant.
If you’re expecting: Tell your baby’s doctor that you took Tocilizumab during your pregnancy. It could impact the baby’s vaccination schedule in the first few months of life. Your name could be included in a pregnancy registry to monitor how tocilizumab affects the baby. the infant.
How to take Tocilizumab?
Take Tocilizumab as directed by your physician. Follow the directions on your prescription label, and go through all the medication guides or instructions sheets.
Before using Tocilizumab your doctor might examine you for tuberculosis or other infections.
Tocilizumab is injected beneath your skin. It can also be injected into the vein. It is usually administered every one to four weeks for all ailments. For CRS, just one dose is typically administered.
In the event of injection into a vein, medicine is administered slowly, for approximately 1 hour.
Follow all instructions for use. Consult your physician or pharmacist should you require assistance.
Only inject when you are prepared to administer it. Inform your pharmacist if your medicine is cloudy, changed color, or has particles.
Your physician will inform you of the best place you should inject Tocilizumab. Don’t inject the same spot twice in the same row.
Don’t reuse needles or autoinjectors. Keep them in an impervious to puncture “sharps” container. Then dispose of them according to the laws of your state or municipality. Keep them out of reach of pets and children.
It is possible to contract infections more often, or even fatal or serious infections. You’ll need regular medical tests.
If you’ve been a victim of hepatitis B and it has returned, it could or become worse. It is possible to require tests for liver function during the course of this treatment and for a few months after stopping.
Inform your doctor if you are planning to undergo a procedure.
Make sure you take all your medicines according to the directions. Do not alter the dose or stop taking the medicine without consulting your doctor.
Storage Tocilizumab injection inside the original carton in the refrigerator. Be sure to keep it away from light and moisture. Avoid freezing. Recycle any prefilled syringes that are not used prior to the expiration date. the end date on the label of the medicine.
Dispose of the autoinjector or syringe you have filled after one-time use even if there’s some medicine in the.
Tocilizumab may have lasting consequences on the human body. It is possible that you will require certain medical tests every six months after stopping taking this drug.
Dosing information
The Usual Adult Dose Of Tocilizumab For Rheumatoid Arthritis:
- IV 4 mg/kg IV single drip infusion lasting 60 minutes every 4 weeks. and then an increase of 8 mg/kg IV every 4 weeks, as a single drip infusion lasting 60 minutes. dependent on the clinical response. doses of more than 800 mg/infusion are not advised.
SUBCUTANEOUS:
Patients less than 100 kg: 162 mg subcutaneously every two weeks and then an increase to every other week dependent on clinical response
Patients who weigh 100 kg or more 160 mg subcutaneously each week - Comments:
This drug can be utilized as a monotherapy, or with methotrexate and various other non-biologic DMARDs.
When you are switching from subcutaneous to IV take the first dose of subcutaneous instead of the dose of IV that follows. - Use: To treat adults suffering from moderately or severely active Rheumatoid Arthritis who have experienced an insufficient response to any one or more Disease-Modifying Anti-Rheumatic Drugs (DMARDs)
Normal Adult Dose Tocilizumab for Large Cell Arteritis
- SUBCUTANEOUS:
In combination with a tapering program of glucocorticoids. 162 mg subcutaneously administered once a week.
Based on the clinical evidence 162 mg of subcutaneously administered every two weeks in conjunction with a tapering program of glucocorticoids could be prescribed. - Comments:
The drug is available in conjunction with glucocorticoids after discontinuation.
Normal Adult Dose of Cytokine-Associated Toxicity
- IV:
Weight less than 30kg 12 mg/kg intravenous over 60 minutes
Weight 30 kg or more 8 mg/kg intravenous over 60 minutes - Comments:
Doses that exceed 800 mg per dose are not recommended.
If there is no improvement in the clinical condition after the first dose three additional doses could be administered.
The time between two doses must be at least 8 hours.
The drug is available as a stand-alone drug or in conjunction with corticosteroids.
Patients suffering from life-threatening CRS typically have cytopenias, or an elevated ALT as well as AST due to the malignancy that caused them prior to chemotherapy for lymphodepletion or CRS.
The subcutaneous route is not approved for CRS.
The usual pediatric dose for juvenile Idiopathic Arthritis
- PJIA, also known as Polyarticular Joint Idiopathic Arthritis (PJIA):
IV formulation 2 years or more
Weigh less than 30 kg 10 mg/kg intravenous over 60 minutes once every four weeks
Weight 30-kg or greater 8 mg/kg intravenous over 60 minutes each week for 4 weeks
SUBCUTANEOUS formulation
Weigh less than 30 kg: 162 mg twice every 3 weeks
Weight 30 kg or greater: 162 mg every two weeks - Systemic Juvenile Idiopathic Arthritis (SJIA):
IV formulation 2 years or more:
Weigh less than 30kg 12 mg/kg IV for 60 minutes every two weeks
If you weigh 30kg or more 8 mg/kg IV for 60 minutes each week for 2 weeks
SubcutaNEOUS formulation:
Weight less than 30kg 162 mg subcutaneously every two weeks
If you weigh at least 30 kilograms Subcutaneously 162 mg every other week - Comments:
The drug is utilized as a monotherapy, or with methotrexate.
Weight can fluctuate, therefore dose adjustments shouldn’t be based solely on one weight measurement.
If you’re changing from subcutaneous IV to IV administer the first subcutaneous dose, not the following IV dose. - Uses:
For the treatment of polyarticular active juvenile Idiopathic Arthritis (PJIA) for patients who are 2 years old and over.
for the treatment of juvenile systemic Idiopathic Arthritis (SJIA) for patients who are 2 years old or older
Usual Dose for Pediatric Cytokine-Associated Toxicity
- IV:
Weight less than 30kg 12 mg/kg intravenous over 60 minutes
Weight of 30 kg or greater 8 mg/kg IV for 60 minutes - Comments:
Doses of more than 800 mg/infusion are not recommended.
If there is no improvement in the clinical condition after the first dose up to three doses could be administered.
The time between two doses must be at least 8 hours.
The drug is available either alone or in combination with corticosteroids.
Patients suffering from life-threatening CRS often have cytopenias or an elevated ALT and AST due to malignancy that is underlying or chemotherapy that precedes lymphodepleting therapy or CRS.
Subcutaneous route not approved for CRS. - Use: To treat the chimeric antigen receptor (CAR) T cells that cause a serious or life-threatening cytokine-release syndrome in children and adults patients who are 2 years old or older
If I do not take the dose?
Contact your doctor for advice in case you missed a dose.
If I take too much?
Get medical attention immediately or contact for help at the Poison Help line at 1-800-222-1222.
What to avoid while taking Tocilizumab?
When you are using Tocilizumab Do not get a “live” vaccine, or you may develop an infection that is serious. The vaccine might not work effectively and might not completely protect you from illness.
- The live vaccines are measles-rubella, mumps (MMR) as well as polio, Typhoid, rotavirus fever, and varicella (chickenpox) as well as Zoster ( shingles) and nasal influenza (influenza) vaccination.
- There is a chance to receive a regular vaccine against influenza as well as an “inactivated” or another vaccine to protect yourself from diseases such as meningitis, hepatitis, pneumonia, shingles, HPV, or Whooping Cough.
- Talk to your doctor prior to obtaining any vaccination.
Avoid being around those that are sick, or suffering from infections. Inform your doctor immediately when you begin to show symptoms of an infection.
Tocilizumab side effects
Take immediate medical attention If you are experiencing symptoms that you are experiencing an allergic reaction with Tocilizumab, such as itching or chest pains; difficulties breathing, feeling like you’re about to faint or pass out; swelling of your lips, face, and tongue.
Consult your physician immediately If you suffer from:
- extreme stomach cramps and bloating, the sensation of bloating, diarrhea, or constipation;
- bleeding that is unusual abnormal bleeding nosebleeds bleeding gums, unusual vaginal bleeding or any bleeding that doesn’t stop bleeding in your stool or urine and coughing up blood, or vomit that looks similar to coffee grounds
- liver disorders Loss of appetite, stomach pain on the right side vomiting, confusion dark urine, dark stools, jaundice (yellowing of the eyes or skin);
- symptoms of signs such as chills, fever, and aches as well as diarrhea, skin sores burning sensation when you go to the bathroom;
- symptoms of tuberculosis include cough, breathlessness, night sweats, and loss of appetite. loss of weight, and fatigue or
- symptoms of perforation (a tear or hole) in the stomach or intestines (fever, constant stomach pain, changes in your bowel habits.
The most common Tocilizumab-related side effects can include:
- Runny or stuffy nose sinus discomfort, sore throat;
- headache;
- elevated blood pressure
- an abnormal tests for liver function abnormal liver function tests
- inflammation, pain, swelling, or irritation where an injection was administered.
This isn’t an exhaustive list of all side effects. other side effects could be present. Contact your doctor for advice regarding medical the effects. You can report symptoms to FDA at 1-800-FDA-1088.
What other medications can interfere with Tocilizumab?
It is sometimes not safe to take certain medications in conjunction. Certain drugs may affect the blood levels and levels of other drugs that you are taking. This could cause more side effects or make the drugs less effective.
Inform your doctor about the other medications you take including other medications to combat rheumatoid arthritis-like:
- abatacept, etanercept;
- anakinra or
- adalimumab, certolizumab, golimumab, infliximab, or rituximab.
This isn’t a complete list and other medications may be incompatible with tocilizumab. This includes prescription and over-the-counter drugs, vitamins, and herbal products. The interactions of all drugs are listed here.
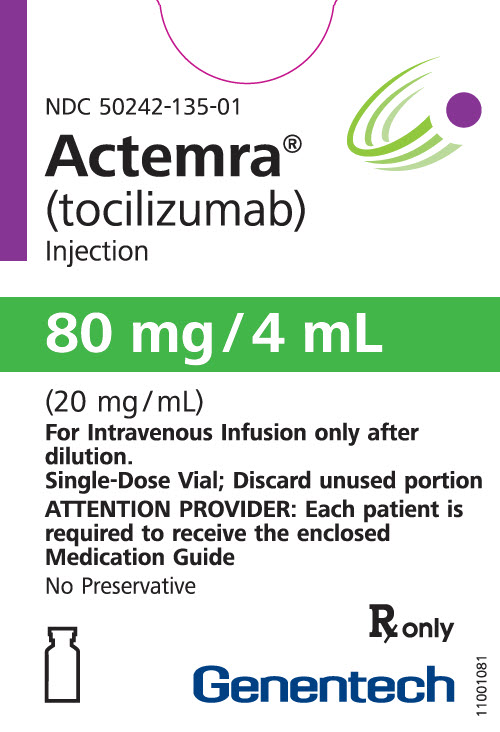
How is Actemra supplied
4 ML Vial Box
- 80 mg/4 mL (20 mg/mL)
- For IV use
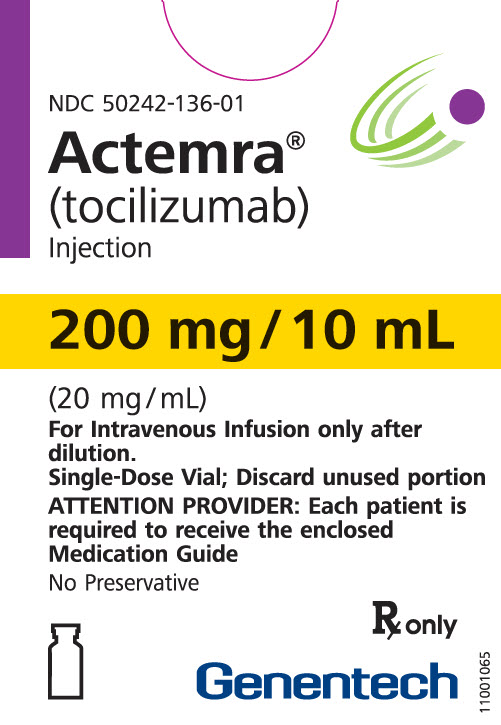
10 ml Vial Box
- 200 mg/10 mL
- (20 mg/mL)
- Rx only
More details
Keep all medicines away from the reach of children. Do not let your medications be shared with anyone else and only use Tocilizumab to treat the condition prescribed.
Always consult your doctor to make sure the information presented on this site is appropriate to your particular situation.
