TOBI Podhaler
Generic name: tobramycin (inhalation)
Brand names: Bethkis, Kitabis Pak, Tobi, Tobi Podhaler
Drug classes: Aminoglycosides, Inhaled anti-infectives
Medically reviewed by A Ras MD.
What is TOBI Podhaler?
TOBI Podhaler is a prescription medicine used to treat people with cystic fibrosis who have a bacterial infection called Pseudomonas aeruginosa. TOBI Podhaler contains an antibacterial medicine called tobramycin (an aminoglycoside).
It is not known if TOBI Podhaler is safe and effective:
- in children under 6 years of age
- in people who have an FEV1 less than 25% or greater than 80% predicted
- in people who are colonized with a bacterium called Burkholderia cepacia
Description
TOBI Podhaler consists of a dry powder formulation of tobramycin for oral inhalation only with the Podhaler device. The inhalation powder is filled into clear, colorless hypromellose capsules.
Each clear, colorless hypromellose capsule contains a spray dried powder of 28 mg of tobramycin active ingredient with 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), calcium chloride, and sulfuric acid (for pH adjustment).
The active component of TOBI Podhaler is tobramycin. Tobramycin is an aminoglycoside antibiotic. Its chemical name is O-3-amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6-trideoxy-α-D-ribo-hexopyranosyl-(1→6)]-2-deoxy-L-streptamine; its structural formula is:

Tobramycin has a molecular weight of 467.52, and its empirical formula is C18H37N5O9. Tobramycin is a white to almost white powder; visually free from any foreign contaminants. Tobramycin is freely soluble in water, very slightly soluble in ethanol, and practically insoluble in chloroform and ether.
The Podhaler device is a plastic device used to inhale the dry powder contained in the TOBI Podhaler capsule. Under standardized in vitro testing at a fixed flow rate of 60 L/min and volume of 2 L for 2 seconds, the Podhaler device has a target delivered dose of 102 mg of tobramycin from the mouthpiece (4 capsules per dose).
Peak inspiratory flow rate and inhaled volumes were explored in 96 cystic fibrosis patients aged 6 years and older. Older patients with significant disease progression and associated decreases in forced expiratory volume (FEV1) and younger patients with inhaled volumes <1 L were able to generate inspiratory flow rates and volumes required to receive their medication when following the instructions for use. However, no pediatric patients aged 6 to 10 years with FEV1 less than 40% predicted were evaluated.
What is the most important information I should know about TOBI Podhaler?
Do not swallow TOBI Podhaler capsules. TOBI Podhaler capsules are used only with the Podhaler device and inhaled through your mouth (oral inhalation). Never place a capsule in the mouthpiece of the Podhaler device.
Who should not use TOBI Podhaler?
Do not use TOBI Podhaler if you are allergic to tobramycin, any of the ingredients in TOBI Podhaler, or to any other aminoglycoside antibacterial medicines.
What should I tell my healthcare provider before using TOBI Podhaler?
Before using TOBI Podhaler, tell your healthcare provider about all of your medical conditions, including if you:
- have or have had hearing problems (including noises in your ears such as ringing or hissing).
- have dizziness.
- have or have had kidney problems.
- have or have had problems with muscle weakness such as myasthenia gravis or Parkinson’s disease.
- have or have had breathing problems such as wheezing, coughing, or chest tightness.
- have had an organ transplant.
- are pregnant or plan to become pregnant. TOBI Podhaler contains a medicine that can harm your unborn baby. See “What are the possible side effects of TOBI Podhaler?” for more information.
- are breastfeeding or plan to breastfeed. It is not known if the medicine in TOBI Podhaler (tobramycin inhalation powder) passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Using TOBI Podhaler with certain other medicines can cause serious side effects. Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
If you are using TOBI Podhaler, you should discuss with your healthcare provider if you should take:
- other medicines that may harm your nervous system, kidneys, or hearing
- “water pills” (diuretics) such as Edecrin (ethacrynic acid), Lasix (furosemide), or intravenous mannitol
- urea
How should I use TOBI Podhaler?
- See the step-by-step Instructions for Use that come with the TOBI Podhaler about the right way to use TOBI Podhaler. Do not use TOBI Podhaler unless your healthcare provider has taught you how to use it the right way.
- Use TOBI Podhaler exactly as your healthcare provider tells you to use it. Ask your healthcare provider or pharmacist if you are not sure.
- The usual dose for adults and children over 6 years of age is:
- The contents of 4 TOBI Podhaler capsules inhaled by mouth in the morning using your Podhaler device and the contents of 4 TOBI Podhaler capsules inhaled by mouth in the evening using your Podhaler device.
- Check to see that each capsule is empty after inhaling. If powder remains in the capsule, repeat inhalation until the capsule is empty.
- Each dose of 4 TOBI Podhaler capsules should be taken as close to 12 hours apart as possible.
- You should not take your dose of 4 TOBI Podhaler capsules less than 6 hours apart.
- After using TOBI Podhaler for 28 days, you should stop using it and wait 28 days. After you have stopped using TOBI Podhaler for 28 days, you should start using TOBI Podhaler again for 28 days. Complete the full 28-day course even if you are feeling better. It is important that you keep to the 28-day on, 28-day off cycle (See Figure A).
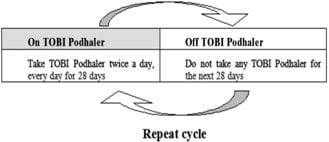
(Figure A) - If you have been prescribed a 7-day pack of TOBI Podhaler either immediately before or during a 28-day treatment with TOBI Podhaler, then you must count each day of use toward the 28-day on-treatment part of the cycle. You should only take a total of 28 consecutive days of treatment during a cycle.
- If you have been prescribed a 1-day pack of TOBI Podhaler either immediately before or during a 28-day treatment with TOBI Podhaler, then you must count each day of use toward the 28-day on-treatment part of the cycle. You should only take a total of 28 consecutive days of treatment during a cycle.
- If you are taking other medicines inhaled through your mouth, your healthcare provider will tell you how to take your medicines the right way.
- If you are doing therapies for cystic fibrosis (chest physiotherapy), you should use TOBI Podhaler after your other therapies are done.
- If you inhale too much TOBI Podhaler, tell your healthcare provider right away.
- If you accidentally swallow TOBI Podhaler capsules, tell your healthcare provider right away.
- Use a new TOBI Podhaler device every 7 days.
- Caregivers should help children who are 10 years of age and younger use TOBI Podhaler, and keep watching them use their TOBI Podhaler until they are able to use it the right way without help.
- Tell your doctor if your symptoms worsen while using your TOBI Podhaler.
What are the possible side effects of TOBI Podhaler?
TOBI Podhaler can cause serious side effects, including:
- severe breathing problems (bronchospasm). Tell your healthcare provider right away if you get any of these symptoms of bronchospasm while using TOBI Podhaler:
- shortness of breath with wheezing
- coughing and chest tightness
- hearing loss or ringing in the ears (ototoxicity). Tell your healthcare provider right away if you have hearing loss or hear noises in your ears such as ringing or hissing, or if you develop vertigo, difficulty with balance or dizziness.
- worsening kidney problems (nephrotoxicity). TOBI Podhaler is in a class of medicines which may cause worsening kidney problems, especially in people with known or suspected kidney problems. Your healthcare provider may do a blood test to check how your kidneys are working while you are using TOBI Podhaler.
- worsening muscle weakness. TOBI Podhaler is in a class of medicines which can cause muscle weakness to get worse in people who already have problems with muscle weakness (myasthenia gravis or Parkinson’s disease).
- The medicine in TOBI Podhaler is in a class of medicines which may cause harm to an unborn baby.
The most common side effects of TOBI Podhaler include:
- cough
- worsening of lung problems or cystic fibrosis
- productive cough
- shortness of breath
- fever
- sore throat
- changes in your voice (hoarseness)
- coughing up blood
- headache
Let your healthcare provider know if your symptoms get worse. Some patients may not be able to continue using TOBI Podhaler and need to consider other treatments. Tell your healthcare provider about any side effect that bothers you enough to stop treatment or that does not go away.
These are not all of the possible side effects of TOBI Podhaler. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of TOBI Podhaler
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TOBI Podhaler for a condition for which it was not prescribed. Do not give TOBI Podhaler to other people, even if they have the same problem you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about TOBI Podhaler that was written for healthcare professionals.
For more information, go to www.TOBIPodhaler.com or call 1-877-999-TOBI (8624).
What are the ingredients in TOBI Podhaler?
Active ingredient: tobramycin
Inactive ingredients: 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), calcium chloride, and sulfuric acid (for pH adjustment)
SRC: NLM .
