Tibsovo
Generic name: ivosidenib
Drug class: Miscellaneous antineoplastics
Medically reviewed by A Ras MD.
What is Tibsovo?
Tibsovo is a prescription medicine used to treat acute myeloid leukemia (AML) with an isocitrate dehydrogenase-1 (IDH1) mutation in:
- adults with newly diagnosed AML who are 75 years or older or who have health problems that prevent the use of certain chemotherapy treatments.
- adults with AML when the disease has come back or has not improved after previous treatment(s).
Your healthcare provider will perform a test to make sure that Tibsovo is right for you.
It is not known if Tibsovo is safe and effective in children.
Description
TIBSOVO (ivosidenib) is an inhibitor of isocitrate dehydrogenase 1 (IDH1) enzyme. The chemical name is (2S)-N-{(1S)-1-(2-chlorophenyl)-2-[(3,3-difluorocyclobutyl)-amino]-2-oxoethyl}-1-(4-cyanopyridin-2-yl)-N-(5-fluoropyridin-3-yl)-5-oxopyrrolidine-2-carboxamide.
The chemical structure is:
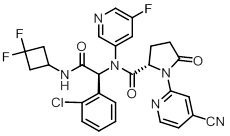
The molecular formula is C28H22C1F3N6O3 and the molecular weight is 583.0 g/mol. Ivosidenib is practically insoluble in aqueous solutions between pH 1.2 and 7.4.
TIBSOVO (ivosidenib) is available as a film-coated 250 mg tablet for oral administration. Each tablet contains the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The tablet coating includes FD&C blue #2, hypromellose, lactose monohydrate, titanium dioxide, and triacetin.
Mechanism of Action
Ivosidenib is a small molecule inhibitor that targets the mutant isocitrate dehydrogenase 1 (IDH1) enzyme. Susceptible IDH1 mutations are defined as those leading to increased levels of 2-hydroxyglutarate (2-HG) in the leukemia cells and where efficacy is predicted by 1) clinically meaningful remissions with the recommended dose of ivosidenib and/or 2) inhibition of mutant IDH1 enzymatic activity at concentrations of ivosidenib sustainable at the recommended dosage according to validated methods. The most common of such mutations are R132H and R132C substitutions.
Ivosidenib was shown to inhibit selected IDH1 R132 mutants at much lower concentrations than wild-type IDH1 in vitro. Inhibition of the mutant IDH1 enzyme by ivosidenib led to decreased 2-HG levels and induced myeloid differentiation in vitro and in vivo in mouse xenograft models of IDH1-mutated AML. In blood samples from patients with AML with mutated IDH1, ivosidenib decreased 2-HG levels ex-vivo, reduced blast counts, and increased percentages of mature myeloid cells.
What is the most important information I should know about Tibsovo?
Tibsovo may cause serious side effects, including:
- Differentiation Syndrome. Differentiation syndrome is a condition that affects your blood cells and may be life-threatening or lead to death if not treated. Differentiation syndrome has happened as early as 1 day and up to 3 months after starting Tibsovo. Call your healthcare provider or go to the nearest hospital emergency room right away if you develop any of the following symptoms of differentiation syndrome while taking Tibsovo:
- fever
- cough
- trouble breathing
- rash
- decreased urination
- dizziness or lightheadedness
- rapid weight gain
- swelling of your arms or legs
If you develop signs and symptoms of differentiation syndrome, your healthcare provider may treat you with a corticosteroid medicine or a medicine called hydroxyurea and may monitor you in the hospital.
See “What are the possible side effects of Tibsovo?” for more information about side effects.
What should I tell my healthcare provider before taking Tibsovo?
Before taking Tibsovo, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including a condition called long QT syndrome.
- have problems with abnormal electrolytes, such as sodium, potassium, or magnesium levels.
- have nervous system problems.
- have problems with your kidneys or are on dialysis.
- have any liver disorders, including cirrhosis.
- are pregnant or plan to become pregnant. Tibsovo may cause harm to your unborn baby. You should avoid becoming pregnant during treatment with Tibsovo. Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with Tibsovo.
- are breastfeeding or plan to breastfeed. It is not known if Tibsovo passes into your breast milk. Do not breastfeed during your treatment with Tibsovo and for at least 1 month after your last dose of Tibsovo.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take hormonal contraceptives. Tibsovo may affect how hormonal contraceptives work and may cause them to not work as well.
How should I take Tibsovo?
- Take Tibsovo exactly as your healthcare provider tells you to.
- Do not change your dose or stop taking Tibsovo without talking to your healthcare provider.
- Take Tibsovo 1 time a day about the same time each day.
- Swallow Tibsovo tablets whole. Do not split, crush, or chew the tablet.
- Tibsovo can be taken with or without food.
- Do not take Tibsovo with a high-fat meal. An example of a high-fat meal includes 2 eggs fried in butter, 2 strips of bacon, 2 slices of white bread with butter, 1 croissant with 1 slice of cheese, and 8 ounces of whole milk (approximately 1,000 calories and 58 grams of fat).
- If you vomit after taking a dose of Tibsovo, do not take an additional dose. Take your next dose at your usual time.
- If you miss a dose of Tibsovo or did not take it at the usual time, take your dose as soon as possible and at least 12 hours before your next dose. Return to your normal schedule the following day. Do not take 2 doses of Tibsovo within 12 hours.
What are the possible side effects of Tibsovo?
Tibsovo may cause serious side effects, including:
- See “What is the most important information I should know about Tibsovo?”
- Changes in the electrical activity of your heart called QTc prolongation. QTc prolongation can cause irregular heartbeats that can be life-threatening. Your healthcare provider will check the electrical activity of your heart with a test called an electrocardiogram (ECG) during treatment with Tibsovo. Tell your healthcare provider right away if you feel dizzy, lightheaded, or faint.
- Guillain-Barré syndrome has happened in people treated with Tibsovo. Your healthcare provider will monitor you for nervous system problems and will permanently stop your treatment with Tibsovo if you develop Guillain-Barré syndrome. Tell your healthcare provider right away if you develop any signs or symptoms of Guillain-Barré syndrome, including:
- weakness or tingling feeling in your legs, arms, or upper body
- numbness and pain on one side or both sides of your body
- any changes in your ability to see, touch, hear, or taste
- burning or prickling sensation
- difficulty breathing
The most common side effects of Tibsovo include:
- fatigue
- joint pain
- high white blood cell count
- diarrhea
- swelling of arms or legs
- nausea
- shortness of breath
- pain or sores in your mouth or throat
- irregular heart rhythm or heartbeat (QTc prolongation)
- rash
- cough
- decreased appetite
- muscle pain
- constipation
- fever
- hemoglobin decreased (anemia)
- decreased levels of electrolytes in the blood
- changes in liver or kidney function tests
Your healthcare provider will do blood tests before you start and during treatment with Tibsovo. Your healthcare provider may decrease, temporarily hold, or permanently stop your treatment with Tibsovo if you develop side effects.
Tibsovo may cause fertility problems in females and males, which may affect your ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of Tibsovo.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Tibsovo
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take Tibsovo for conditions for which it was not prescribed. Do not give Tibsovo to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Tibsovo that is written for healthcare professionals.
How should I store Tibsovo?
- Store Tibsovo at room temperature between 68°C to 77°C (20°C to 25°C).
Keep Tibsovo and all medicines out of the reach of children.
What are the ingredients in Tibsovo?
Active ingredient: ivosidenib
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, magnesium stearate, microcrystalline cellulose, and sodium lauryl sulfate. The tablet coating includes FD&C blue #2, hypromellose, lactose monohydrate, titanium dioxide, and triacetin.
Label
PRINCIPAL DISPLAY PANEL – NDC: 71334-100-01 – 250 MG TABLET 60-COUNT BOTTLE LABEL

