Tezspire Dosage
- Generic Name: tezepelumab-ekko
- Dosage Form: injection, for subcutaneous use
- Medically reviewed by R Shah MD. Updated on March 20, 2022.
What is Tezspire?

Tezspire is a human monoclonal antibody immunoglobulin G2 (IgG2) generated in Chinese hamster ovary (CHO) cells using recombinant DNA technology. It is a thymic stromal lymphopoietin (TSLP) blocker. The molecular weight of tezepelumab-ekko is approximately 147 kDa.
Tezspire (tezepelumab-ekko) injection is a single-dose vial or single-dose pre-filled syringe of sterile, preservative-free, clear to opalescent, colorless to light yellow solution for subcutaneous injection.
Each single-dose vial or pre-filled syringe contains 1.91 mL of tezepelumab-ekko, 2.8 mg of glacial acetic acid, 48 mg of L-proline, 0.19 mg of polysorbate 80, sodium hydroxide, and water for injection. The pH of the water is 5.2.
Tezspire is a prescribed medicine that is used in conjunction with other asthma medications to treat severe asthma in patients who are 12 years old or older whose asthma isn’t controlled by their current asthma medication.
Tezspire is a great way to stop serious asthma attacks (exacerbations) and improves your breathing.
Tezspire is not intended to treat breathing issues that arise suddenly. Inform your doctor when your asthma doesn’t improve or becomes worse following the start of treatments with Tezspire.¶
It isn’t known whether this medication can be used safely and effectively for children younger than 12 years old. age.¶
Who should not receive Tezspire?
Do not get this medication if:
- Are allergic to tezepelumab or any other ingredients. Look at the end of this page for a full listing of the ingredients.
Prior to receiving Tezspire
Before you get this medication, inform your healthcare provider of the medical conditions you suffer from for example if you
- Have you ever experienced an extremely severe allergic reaction (hypersensitivity).
- are suffering from an infection called a parasite (helminth) disease.
- Recently, or recently, they have received or are scheduled to receive any live vaccines attenuated to live. The Tezspire vaccine is recommended for those who have not received live vaccines attenuated to the point of inactivity.
- If you are pregnant, it is possible that you could be pregnant or planning to be expecting. It’s unclear whether Tezspire can affect your baby’s development.
- If you are nursing or planning to or plan to. It’s not clear whether Tezspire gets into your milk. Discuss with your healthcare professional the best method to feed your child if you are receiving Tezspire.
Mechanism of Action
Tezepelumab-ekko is a thymic stromal lymphopoietin (TSLP) blocker, a human monoclonal antibody IgG2 that binds to human TSLP and blocks its interaction with the heterodimeric TSLP receptor with a dissociation constant of 15.8 pM. TSLP is a cytokine produced mostly by epithelial cells that has a role in the asthma inflammatory cascade.
Inflammation of the airways is a key factor in the development of asthma. Airway inflammation is caused by a variety of cell types (mast cells, eosinophils, neutrophils, macrophages, lymphocytes, and ILC2 cells), as well as mediators (histamine, eicosanoids, leukotrienes, and cytokines). Blocking TSLP with tezepelumab-ekko lowers inflammatory biomarkers and cytokines like blood eosinophils, airway submucosal eosinophils, IgE, FeNO, IL-5, and IL-13; however, the mechanism of tezepelumab-ekko action in asthma has yet to be determined.
What other drugs can impact Tezspire?
Discuss with your doctor the medications you are taking such as prescription and over-the-counter supplements including vitamins, supplements, and herbal supplements. Do not alter or stop taking corticosteroid medications or other asthma medicine unless your doctor advises you to.
How do I get Tezspire?
- Your doctor will offer your Tezspire in a hospital setting.
- Tezspire is injected into the skin (subcutaneously) one time every 4 weeks.
- If you do not make the appointment, you should inform your doctor when you can schedule your next appointment.
Tezspire Dosage
Recommended Dosage
The recommended dose of Tezspire can be 210 milligrams, which is administered subcutaneously every 4 weeks.
Missed Dose Information
If you miss a dose If a dose is missed, administer it promptly. After that, the patient can continue (resume) dosage on the day of the dose. In the event that the dose for next is due, administer the dose as per the plan.
Preparation and Administration Instructions
Tezspire is designed for administration by a healthcare professional.
Each vial and syringe that is pre-filled includes a single dose Tezspire .
Before administering, take Tezspire out of the fridge and let it cool to the temperature of the room. This usually takes around 60 minutes. Avoid exposing to heat or shaking. Don’t apply the container if the security seal on the carton has damaged.
Do not place the carton back into the refrigerator after Tezspire is at the temperature of the room. Once removed from refrigerators, the Tezspire needs to be consumed within 30 days or removed
Check Tezspire for any particulate matter or discoloration prior to the administration. Tezspire is a clear transparent, opalescent, colorless to mild yellow liquid. Do not use Tezspire when it is cloudy or discolored or has massive particles or other foreign matter. Avoid using the vial, if the filled syringe is damaged or dropped, or if the expiration date is passed.
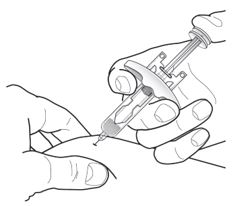
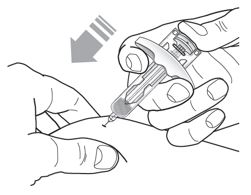
Inject Tezspire at 210 mg (contents of one vial or one syringe that has been pre-filled as explained below) subcutaneously in the upper arm, the thigh, or abdomen with the exception of two inches (5 cm) around the navel. Tezspire should not go in areas where the skin appears fragile or swollen, erythematous, or has become hardened. It is suggested to rotate the site of injection after each injection.
Administration Instructions for Single-Dose Pre-filled Syringe
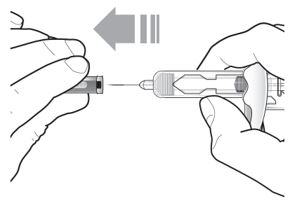
Look at Figure 1 to determine the components of the syringe pre-filled for the administration procedures.
Do not take off your needle cap until the second step of these steps when you are ready to inject Tezspire . Don’t contact the activation clips for the needle guard to avoid unintentional activation of the needle security guard.
Figure Tezspire Pre-filled Syringe Components
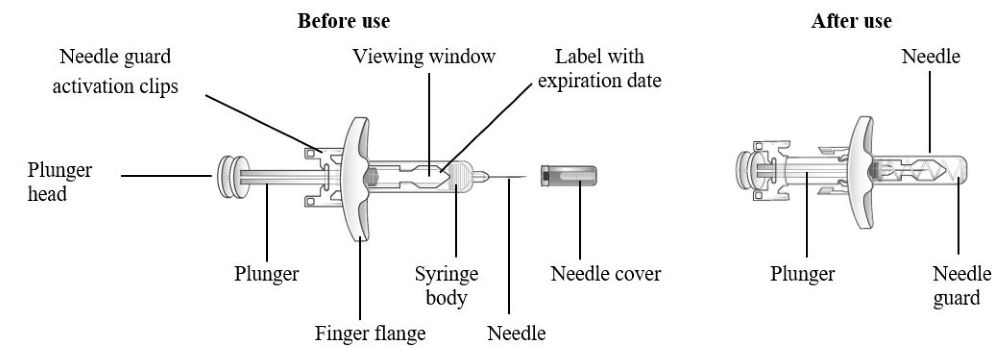
- Grasp the syringe body to remove the pre-filled syringe from its tray. Do not grab the pre-filled syringe by the plunger.
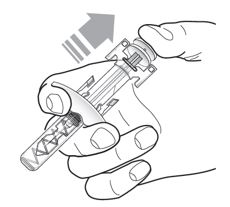
- The pre-filled syringe may contain small air bubbles; this is normal. Do not expel the air bubbles prior to administration.
6. Recycle the syringe and place it in Sharps containers.
How Supplied
The injections are a preservative-free, sterile clear to opalescent clear to light yellow, colorless solution that is available as a single-dose vial or single-dose vial with a 27 gauge fixed 1/2 inch needle, with needle covers. The cap for the needle and vial stopper is not made from natural latex rubber.
This is available in the following formats:
- Single-Dose vial: Carton has a dose of 210 mg/1.91 milliliters (110 mg/mL) in a glass vial (NDC 55513-100-01)
- Single-Dose Syringe Pre-filled: Carton contains one 210 mg/1.91 milliliter (110 mg/mL) pre-filled Syringe (NDC 55513-112-01)
Storage and Handling
Store in a cool, dry place between 36degF and 46degF (2degC to 8 degC). If needed, Tezspire may be kept at room temperature, ranging from 68degF and 77@F (20degC between 25 and 25degC) for up to 30 days. Don’t put it back in the refrigerator until Tezspire is at temperatures of room temperature. Once removed from the refrigerator Tezspire is to be utilized within 30 days or removed from the refrigerator.
Keep Tezspire in the original packaging to keep it safe from sunlight until the time of use.
Avoid freezing. Don’t shake. Avoid exposure to heat.
Label

More details
Always consult your doctor to make sure the information presented on this page is applicable to your particular situation.