Skyrizi
Generic name: risankizumab
Drug class: Interleukin inhibitors
Medically reviewed by A Ras MD.
What is Skyrizi?
Skyrizi is a prescription medicine used to treat adults with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
It is not known if Skyrizi is safe and effective in children under 18 years of age.
Description
Risankizumab-rzaa, an interleukin-23 (IL-23) antagonist, is a humanized immunoglobulin G1 (IgG1) monoclonal antibody. Risankizumab-rzaa is produced by recombinant DNA technology in Chinese hamster ovary cells and has an approximate molecular weight of 149 kDa.
SKYRIZI (risankizumab-rzaa) injection 150 mg/mL prefilled syringe or prefilled pen for subcutaneous use
Each SKYRIZI prefilled pen or prefilled syringe contains a sterile, preservative-free, colorless to yellow, and clear to slightly opalescent solution. Each syringe and pen delivers 150 mg of risankizumab-rzaa and the inactive ingredients glacial acetic acid (0.054 mg), polysorbate 20 (0.2 mg), sodium acetate (0.75 mg), trehalose (63.33 mg), and Water for Injection, USP. The pH is 5.7.
SKYRIZI (risankizumab-rzaa) injection 75 mg/0.83 mL prefilled syringe for subcutaneous use
Each SKYRIZI prefilled syringe contains a sterile, preservative-free, colorless to slightly yellow, and clear to slightly opalescent solution. Each syringe delivers 75 mg of risankizumab-rzaa, and the inactive ingredients sodium succinate (0.53 mg), polysorbate 20 (0.17 mg), sorbitol (34 mg), succinic acid (0.049 mg), and Water for Injection, USP. The pH is 6.2.
SKYRIZI (risankizumab-rzaa) injection 360 mg/2.4 mL (150 mg/mL) prefilled cartridge for use with the supplied on-body injector for subcutaneous use
Each SKYRIZI prefilled cartridge contains a sterile, preservative-free, colorless to yellow, and clear to slightly opalescent solution. Each cartridge delivers 360 mg of risankizumab-rzaa, and the inactive ingredients glacial acetic acid (0.13 mg), polysorbate 20 (0.48 mg), sodium acetate (1.8 mg), trehalose (152 mg), and Water for Injection, USP. The pH is 5.7.
SKYRIZI 600 mg/10 mL (60 mg/mL) in a vial for intravenous infusion
SKYRIZI (risankizumab-rzaa) injection 600 mg/10 mL (60 mg/mL) is a sterile, preservative-free, colorless to slightly yellow, and clear to slightly opalescent solution in a 10 mL single-dose vial.
Each 10 mL single-dose vial contains 600 mg of risankizumab-rzaa, and the inactive ingredients glacial acetic acid (0.54 mg), polysorbate 20 (2 mg), sodium acetate (7.5 mg), trehalose (633.3 mg), and Water for Injection, USP. The pH is 5.7.
Mechanism of Action
Risankizumab-rzaa is a humanized IgG1 monoclonal antibody that selectively binds to the p19 subunit of human IL-23 cytokine and inhibits its interaction with the IL-23 receptor. IL-23 is a naturally occurring cytokine that is involved in inflammatory and immune responses.
Risankizumab-rzaa inhibits the release of pro-inflammatory cytokines and chemokines.
What is the most important information I should know about Skyrizi?
Skyrizi may cause serious side effects, including:
Infections. Skyrizi may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with Skyrizi and may treat you for TB before you begin treatment with Skyrizi if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with Skyrizi. Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- cough
- shortness of breath
- blood in your mucus (phlegm)
- muscle aches
- warm, red, or painful skin or sores on your body different from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
See “What are the possible side effects of Skyrizi?” for more information about side effects.
What should I tell my healthcare provider before using Skyrizi?
Before using Skyrizi, tell your healthcare provider about all of your medical conditions, including if you:
- have any of the conditions or symptoms listed in the section “What is the most important information I should know about Skyrizi?”
- have an infection that does not go away or that keeps coming back.
- have TB or have been in close contact with someone with TB.
- have recently received or are scheduled to receive an immunization (vaccine). You should avoid receiving live vaccines during treatment with Skyrizi.
- are pregnant or plan to become pregnant. It is not known if Skyrizi can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Skyrizi passes into your breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use Skyrizi?
See the detailed “Instructions for Use” that comes with Skyrizi for information on how to prepare and inject a dose of Skyrizi, and how to properly throw away (dispose of) used Skyrizi prefilled syringes.
- Use Skyrizi exactly as your healthcare provider tells you to use it.
- If you miss your Skyrizi dose, inject a dose as soon as you remember. Then, take your next dose at your regular scheduled time. Call your healthcare provider if you are not sure what to do.
- If you inject more Skyrizi than prescribed, call your healthcare provider right away.
What are the possible side effects of Skyrizi?
Skyrizi may cause serious side effects. See “What is the most important information I should know about Skyrizi?”
The most common side effects of Skyrizi include:
- upper respiratory infections
- feeling tired
- fungal skin infection
- injection site reactions
- headache
These are not all of the possible side effects of Skyrizi. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Skyrizi
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Skyrizi for a condition for which it was not prescribed. Do not give Skyrizi to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Skyrizi that is written for health professionals.
How should I store Skyrizi?
- Store Skyrizi in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze Skyrizi.
- Do not shake Skyrizi.
- Keep Skyrizi in the original carton to protect it from light.
- Skyrizi is not made with natural rubber latex.
Keep Skyrizi and all medicines out of the reach of children.
What are the ingredients in Skyrizi?
Active ingredient: risankizumab-rzaa
Inactive ingredients: disodium succinate hexahydrate, polysorbate 20, sorbitol, succinic acid, and Water for Injection, USP.
For more information, call 1-866-SKYRIZI (1-866-759-7494) or go to www.SKYRIZI.com.
Instructions for use for Skyrizi
Skyrizi (sky-RIZZ-ee)
(risankizumab-rzaa)
injection, for subcutaneous use
Skyrizi Single-Dose Prefilled Syringe
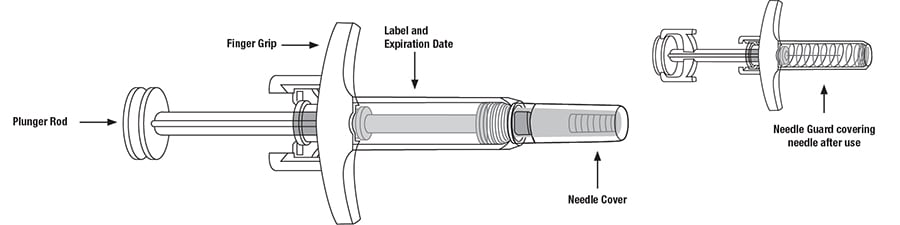
Important Information:
- Keep Skyrizi in the original carton to protect from light until time to use.
- The liquid should look clear to slightly yellow and may contain tiny white or clear particles.
- Do not use Skyrizi if the liquid is cloudy or contains flakes or large particles.
- Do not use Skyrizi if the expiration date (EXP:) shown on the carton and prefilled syringe has passed.
- Do not use Skyrizi if the liquid has been frozen (even if thawed).
- Do not shake Skyrizi.
- Do not use Skyrizi if the syringe has been dropped or damaged.
- Do not use Skyrizi if carton perforations are broken. Return product to the pharmacy.
- Do not remove the needle cover until right before giving the injections.
Keep Skyrizi and all medicines out of the reach of children.
Please Read Complete Instructions For Use Before Using Skyrizi Syringe
Before Injecting:
- Receive training on how to inject Skyrizi before giving injections. Call your healthcare provider or (866) Skyrizi or (866) 759-7494 if you need help.
- Mark your calendar ahead of time to remember when to take Skyrizi.
- Leave the carton at room temperature and out of direct sunlight for 15 to 30 minutes to warm.
- Do not remove the syringes from the carton while allowing Skyrizi to reach room temperature.
- Do not warm Skyrizi in any other way (for example, Do not warm it in a microwave or in hot water).
Important Information:
- The liquid should look clear to slightly yellow and may contain tiny white or clear particles.
- Do not use Skyrizi if the liquid is cloudy or contains flakes or large particles.
- Do not use Skyrizi if the expiration date (EXP:) shown on the carton and prefilled syringe has passed.
- Do not use Skyrizi if the syringe has been dropped or damaged.
- Do not use Skyrizi if carton perforations are broken. Return product to the pharmacy.
Storage Information:
- Store Skyrizi in your refrigerator between 36° F to 46°F (2° to 8°C).
- Do not shake Skyrizi.
- Keep Skyrizi in the original carton to protect from light until time to use.
- Skyrizi is not made with natural rubber latex.
- Do not use if liquid has been frozen (even if thawed).
Keep Skyrizi and all medicines out of the reach of children.
Call your healthcare provider or (866) Skyrizi or (866) 759-7494 if you need help or Do not know how to proceed.

Gather the supplies for the injections and place the following on a clean, flat surface:
- 2 prefilled syringes and 2 alcohol swabs (included)
- 2 cotton balls or gauze pads (not included)
- FDA-cleared sharps disposal container (not included)
Wash and dry your hands.
Start with one prefilled syringe for first injection.

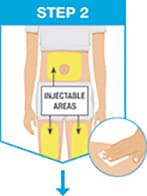
Pick from the 3 injectable areas:
- Front of left thigh or right thigh
- Your abdomen (belly) at least 2 inches from your navel (belly button)

Wipe the injection site in a circular motion with the alcohol swab (before both injections)
- Do not touch or blow on the injection site after it is cleaned. Allow the skin to dry before injecting.
- Do not inject through clothes.
- Do not inject into skin that is sore, bruised, red, hard, scarred, or has stretch marks, or into areas affected by psoriasis.
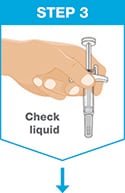
Hold the prefilled syringe with covered needle facing down, as shown.
Check the liquid in the prefilled syringe.
- It is normal to see one or more bubbles in the window.
- The liquid should look clear to slightly yellow and may contain tiny white or clear particles.
- Do not use the prefilled syringe if liquid is cloudy or contains flakes or large particles.
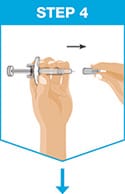
Remove the needle cover.
- Hold the syringe in one hand between the finger grip and needle cover.
- With the other hand, gently pull the needle cover straight off.
- Do not hold or pull plunger rod when removing the needle cover.
- You may see a drop of liquid at the end of the needle. This is normal.
- Throw away the needle cover.
- Do not touch the needle with your fingers or let the needle touch anything.
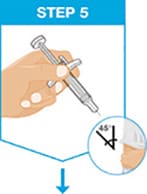
Hold the body of the prefilled syringe in one hand between the thumb and index fingers.
Gently pinch the area of cleaned skin with your other hand and hold it firmly.
Insert the needle into the skin at about a 45-degree angle using a quick, short movement. Hold angle steady.
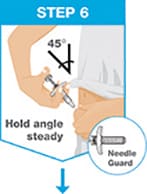
Slowly push the plunger rod all the way in until all of the liquid is injected, and the syringe is empty.
Pull the needle out of the skin while keeping the syringe at the same angle.
Release the plunger rod and allow the prefilled syringe to move up until the entire needle is covered by the needle guard.
The prefilled syringe needle guard will not activate unless all the liquid has been injected.
- Press a cotton ball or gauze pad over the injection site and hold for 10 seconds.
- Do not rub the injection site. You may have slight bleeding. This is normal.

Repeat Steps 2 through 6 with the 2nd prefilled syringe for a full dose.
- Use the 2nd prefilled syringe right after using the 1st syringe.

Put your used prefilled syringes in a FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) used prefilled syringes in the household trash.
For more information, see “Used Skyrizi Prefilled Syringe Disposal” section.
Questions About Using Skyrizi
Q. What if I need help on how to inject Skyrizi?
A. Call your healthcare provider or (866) Skyrizi or (866) 759-7494 if you need help.
Q. What should I do with both used prefilled syringes after my injections?
A. Throw away (dispose of) both used prefilled syringes in a sharps disposal container and not your household trash.
You can sign up to receive sharps containers for Skyrizi syringe disposal at no additional cost by going to www.Skyrizi.com or calling (866) Skyrizi or (866) 759-7494.
Q. How do I know when the injection is complete?
A. The injection is complete when the prefilled syringe is empty, the plunger rod is pushed all the way in, and the syringe needle guard is activated.
Used Skyrizi Prefilled Syringe Disposal
If you Do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: www.fda.gov/safesharpsdisposal.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0074-1050-70
- NOT FOR SALE
- One 1 mL Single-Dose Prefilled Syringe
- Skyrizi® 150 mg/mL
- risankizumab-rzaa Injection
- FOR SUBCUTANEOUS USE ONLY
- Return to pharmacy if carton perforations are broken.
- ATTENTION PHARMACIST:
- Each patient is required to receive
- the enclosed Medication Guide.
- This entire carton is dispensed as a unit.
- www.SKYRIZI.com
- Rx only
- abbvie

PRINCIPAL DISPLAY PANEL
- NDC 0074-2042-02
- 2 x 0.83 mL prefilled syringes
- Skyrizi®
- risankizumab-rzaa Injection
- 75 mg/0.83 mL per Syringe
- FOR SUBCUTANEOUS USE ONLY
- FOR A 150 MG DOSE,
TWO 75 MG SYRINGES ARE REQUIRED,
INJECT ONE SYRINGE AFTER THE OTHER - ATTENTION PHARMACIST:
Dispense the enclosed Medication Guide to each patient. - The entire carton is to be dispensed as a unit.
- Return to pharmacy if carton perforations are broken.
- www.SKYRIZI.com
- Rx only
- abbvie

SRC: NLM .
