Simbrinza
Generic name: brimonidine and brinzolamide ophthalmic
Drug class: Ophthalmic glaucoma agents
What is Simbrinza used for?
Simbrinza is a prescription medicine that is used to treat glaucoma. It is used to lower high eye pressure.
Description
SIMBRINZA (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2% is a fixed combination of a carbonic anhydrase inhibitor and an alpha 2 adrenergic receptor agonist.
Brinzolamide is described chemically as: (R)-(+)-4-Ethylamino-2-(3-methoxypropyl)-3,4-dihydro-2H-thieno [3,2-e]-1,2-thiazine-6-sulfonamide-1,1- dioxide. Its empirical formula is C12H21N3O5S3, and its structural formula is:
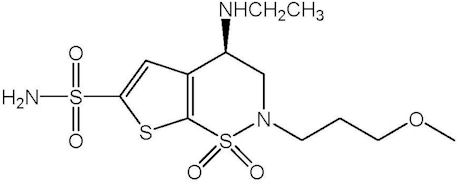
Brinzolamide has a molecular weight of 383.5 g/mol. It is a white powder, which is insoluble in water, very soluble in methanol and soluble in ethanol.
Brimonidine tartrate is described chemically as: 5-bromo-6-(2-imidazolidinylideneamino) quinoxaline L-tartrate. Its empirical formula of C11H10BrN5 – C4H6O6 and its structural formula is:
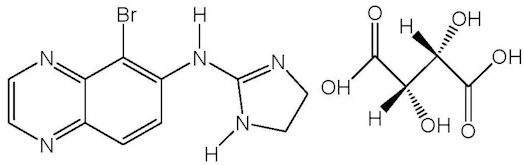
Brimonidine tartrate has a molecular weight of 442.2 g/mol. It is a white to yellow powder that is soluble in water (34 mg/mL) at pH 6.5.
SIMBRINZA (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2% is supplied as a sterile, aqueous suspension which has been formulated to be readily suspended following shaking. It has a pH of approximately 6.5 and an osmolality of approximately 270 mOsm/kg.
Each mL of SIMBRINZA (brinzolamide/brimonidine tartrate ophthalmic suspension) 1%/0.2% contains: Active ingredients: brinzolamide 10 mg, brimonidine tartrate 2 mg (equivalent to 1.32 mg as brimonidine free base); Preservative: benzalkonium chloride 0.03 mg; Inactive ingredients: boric acid, carbomer 974P, mannitol, propylene glycol, purified water, sodium chloride and tyloxapol. Hydrochloric acid and/or sodium hydroxide may be added to adjust pH.
Mechanism of Action
SIMBRINZA is comprised of two components: brinzolamide (carbonic anhydrase inhibitor) and brimonidine tartrate (alpha 2 adrenergic receptor agonist). Each of these two components decreases elevated intraocular pressure. Elevated intraocular pressure is a major risk factor in the pathogenesis of optic nerve damage and glaucomatous visual field loss. The higher the level of intraocular pressure, the greater the likelihood of glaucomatous field loss and optic nerve damage.
Brinzolamide inhibits carbonic anhydrase in the ciliary processes of the eye to decrease aqueous humor secretion, presumably by slowing the formation of bicarbonate ions with subsequent reduction in sodium and fluid transport. Brinzolamide has a peak ocular hypotensive effect occurring at 2 to 3 hours post-dosing. Fluorophotometric studies in animals and humans suggest that brimonidine tartrate has a dual mechanism of action by reducing aqueous humor production and increasing uveoscleral outflow. Brimonidine tartrate has a peak ocular hypotensive effect occurring at two hours post-dosing. The result is a reduction in intraocular pressure (IOP).
Before taking Simbrinza, tell your doctor:
For all patients taking Simbrinza:
- If you are allergic to Simbrinza; any part of this medicine; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had.
- If you have kidney disease.
- If you are taking any of these drugs: Acetazolamide or methazolamide.
- If you are breast-feeding or plan to breast-feed.
Children:
- If your child is younger than 2 years of age. Do not give Simbrinza to a child younger than 2 years of age.
This is not a list of all drugs or health problems that interact with this medicine.
Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take Simbrinza with all of your drugs and health problems. Do not start, stop, or change the dose of any drug without checking with your doctor.
What are some things I need to know or do while I take Simbrinza?
- Tell all of your health care providers that you take Simbrinza. This includes your doctors, nurses, pharmacists, and dentists.
- Avoid driving and doing other tasks or actions that call for you to be alert or have clear eyesight until you see how Simbrinza affects you.
- Have your blood pressure checked often if you have heart disease.
- Have your eye pressure and eyesight checked as you have been told by the doctor.
- Talk with your doctor before you drink alcohol or use other drugs and natural products that slow your actions.
- Tell your doctor if you have an eye infection, eye injury, or will be having eye surgery.
- Tell your doctor if you are pregnant or plan on getting pregnant. You will need to talk about the benefits and risks of using Simbrinza while you are pregnant.
How is Simbrinza best taken?
Use Simbrinza as ordered by your doctor. Read all information given to you. Follow all instructions closely.
- For the eye only.
- Shake well before use.
- Take out contact lenses before using Simbrinza. Lenses may be put back in 15 minutes after Simbrinza is given. Do not put contacts back in if your eyes are irritated or infected.
- Do not touch the container tip to the eye, lid, or other skin.
- Tilt your head back and drop drug into the eye.
- After use, keep your eyes closed. Put pressure on the inside corner of the eye. Do this for 1 to 2 minutes. This keeps the drug in your eye.
- If more than 1 drug is being used in the same eye, use each drug at least 5 minutes apart.
- Do not use if the solution is cloudier than usual, leaking, or has particles.
- Do not use if the liquid changes color.
What do I do if I miss a dose?
- Use a missed dose as soon as you think about it.
- If it is close to the time for your next dose, skip the missed dose and go back to your normal time.
- Do not use 2 doses at the same time or extra doses.
What are the side effects of Simbrinza that I need to call my doctor about immediately?
WARNING/CAUTION: Even though it may be rare, some people may have very bad and sometimes deadly side effects when taking a drug. Tell your doctor or get medical help right away if you have any of the following signs or symptoms that may be related to a very bad side effect:
- Signs of an allergic reaction, like rash; hives; itching; red, swollen, blistered, or peeling skin with or without fever; wheezing; tightness in the chest or throat; trouble breathing, swallowing, or talking; unusual hoarseness; or swelling of the mouth, face, lips, tongue, or throat.
- Change in eyesight, eye pain, or very bad eye irritation.
- If bright lights bother your eyes.
- Rarely, very bad effects have happened with sulfa drugs. Sometimes, these have been deadly. These effects have included liver problems, blood problems, and very bad skin reactions (Stevens-Johnson syndrome/toxic epidermal necrolysis). Call your doctor right away if you have a rash; red, swollen, blistered, or peeling skin; red or irritated eyes; sores in your mouth, throat, nose, or eyes; fever, chills, or sore throat; cough that is new or worse; feeling very tired or weak; any bruising or bleeding; or signs of liver problems like dark urine, feeling tired, not hungry, upset stomach or stomach pain, light-colored stools, throwing up, or yellow skin or eyes.
What are some other side effects of Simbrinza?
All drugs may cause side effects. However, many people have no side effects or only have minor side effects. Call your doctor or get medical help if any of these side effects or any other side effects bother you or do not go away:
- Blurred eyesight.
- Eye irritation.
- Dry mouth.
- Change in taste.
- Burning.
- Stinging.
- Headache.
- Feeling that something is in the eye.
- Feeling sleepy.
These are not all of the side effects that may occur. If you have questions about side effects, call your doctor. Call your doctor for medical advice about side effects.
You may report side effects to the FDA at 1-800-332-1088. You may also report side effects at https://www.fda.gov/medwatch.
If overdose is suspected:
If you think there has been an overdose, call your poison control center or get medical care right away. Be ready to tell or show what was taken, how much, and when it happened.
How do I store and/or throw out Simbrinza?
- Store liquid (solution) in the original container at room temperature. Keep the cap tightly closed. Throw away when the date on the bottle has been reached.
- Keep all drugs in a safe place. Keep all drugs out of the reach of children and pets.
- Throw away unused or expired drugs. Do not flush down a toilet or pour down a drain unless you are told to do so. Check with your pharmacist if you have questions about the best way to throw out drugs. There may be drug take-back programs in your area.
Label
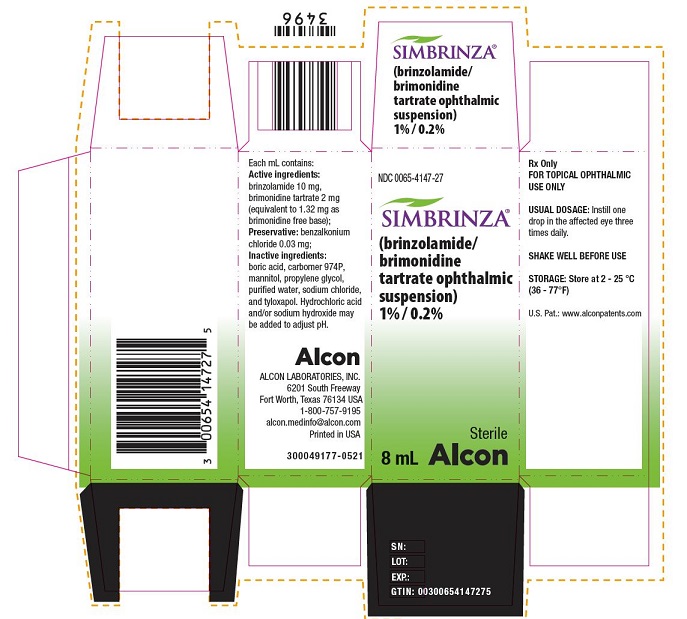
PRINCIPAL DISPLAY PANEL
- NDC 0065-4147-27SIMBRINZA® (brinzolamide/brimonidine tartrate ophthalmic suspension) 1% / 0.2% Sterile 8 mL
Alcon
Rx Only
FOR TOPICAL OPHTHALMIC USE ONLYUSUAL DOSAGE: Instill one
drop in the affected eye three
times daily.SHAKE WELL BEFORE USE
STORAGE: Store at 2 – 25 °C
(36 – 77°F)U.S. Pat.: www.alconpatents.com
SRC: NLM .
