Savaysa
Generic name: edoxaban tosylate
What is Savaysa?
Savaysa is a prescription medicine used to:
- reduce the risk of stroke and blood clots in people who have atrial fibrillation not caused by a heart valve problem.
- treat blood clots in the veins of your legs (deep vein thrombosis) or lungs (pulmonary embolism), after you have been treated with an injectable blood thinner medicine for 5 to 10 days.
It is not known if Savaysa is safe and effective in children.
Description
Edoxaban, a factor Xa inhibitor, is supplied as edoxaban tosylate monohydrate. The chemical name is N-(5-Chloropyridin-2-yl)-N’-[(1S,2R,4S)-4-(N,N-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxamido)cyclohexyl] oxamide mono (4-methylbenzenesulfonate) monohydrate. Edoxaban tosylate monohydrate has the empirical formula C24H30ClN7O4S∙C7H8O3S∙H2O representing a molecular weight of 738.27. The chemical structure of edoxaban tosylate monohydrate is:
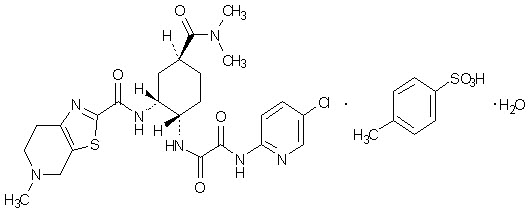
It is a white to pale yellowish-white crystalline powder. The solubility of edoxaban tosylate (pKa 6.7) decreases with increasing pH. It is slightly soluble in water, pH 3 to 5 buffer, very slightly soluble at pH 6 to 7; and practically insoluble at pH 8 to 9.
SAVAYSA is available for oral administration as a 60 mg, 30 mg, or 15 mg round shaped, film-coated tablet, debossed with product identification markings. Each 60 mg tablet contains 80.82 mg edoxaban tosylate monohydrate equivalent to 60 mg of edoxaban. Each 30 mg tablet contains 40.41 mg edoxaban tosylate monohydrate equivalent to 30 mg of edoxaban. Each 15 mg tablet contains 20.20 mg edoxaban tosylate monohydrate equivalent to 15 mg of edoxaban.
The inactive ingredients are: mannitol, pregelatinized starch, crospovidone, hydroxypropyl cellulose, magnesium stearate, talc, and carnauba wax. The color coatings contain hypromellose, titanium dioxide, talc, polyethylene glycol 8000, iron oxide yellow (60 mg tablets and 15 mg tablets), and iron oxide red (30 mg tablets and 15 mg tablets).
Mechanism of Action
Edoxaban is a selective inhibitor of FXa. It does not require antithrombin III for antithrombotic activity. Edoxaban inhibits free FXa, and prothrombinase activity and inhibits thrombin-induced platelet aggregation. Inhibition of FXa in the coagulation cascade reduces thrombin generation and reduces thrombus formation.
What is the most important information I should know about Savaysa?
- For people who take Savaysa for nonvalvular atrial fibrillation (a type of irregular heartbeat):
People with nonvalvular atrial fibrillation are at an increased risk of forming a blood clot in the heart, which can travel to the brain, causing a stroke, or to other parts of the body. Savaysa reduces your risk of having a stroke by helping to prevent clots from forming.- Your doctor should check your kidney function before you start taking Savaysa. People whose kidneys work really well should not receive Savaysa because it may not work well to prevent stroke.
- Do not stop taking Savaysa without first talking to the doctor who prescribed it for you. Stopping Savaysa increases your risk of having a stroke.
- Savaysa can cause bleeding which can be serious, and sometimes lead to death. This is because Savaysa is a blood thinner medicine that reduces blood clotting. During treatment with Savaysa, you may bleed more easily, bleed longer, or bruise more easily. Call your doctor or get medical help right away if you experience bleeding that is severe (for example, coughing up or vomiting blood) or bleeding that cannot be controlled.
You may have a higher risk of bleeding if you take Savaysa and take other medicines that increase your risk of bleeding, including:- aspirin or aspirin containing products
- long-term (chronic) use of non-steroidal anti-inflammatory drugs (NSAIDs)
- long-term (chronic) use of blood thinner medicines, such as:
- warfarin sodium (Coumadin, Jantoven)
- any medicine that contains heparin
- selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs)
- other medicines to prevent or treat blood clots
Tell your doctor if you take any of these medicines. Ask your doctor or pharmacist if you are not sure if your medicine is one listed above.
- Spinal or epidural blood clots (hematoma). People who take a blood thinner medicine (anticoagulant) like Savaysa, and have medicine injected into their spinal and epidural area, or have a spinal puncture have a risk of forming a blood clot that can cause long-term or permanent loss of the ability to move (paralysis). Your risk of developing a spinal or epidural blood clot is higher if:
- a thin tube called an epidural catheter is placed in your back to give you certain medicine
- you take NSAIDs or a medicine to prevent blood from clotting
- you have a history of difficult or repeated epidural or spinal punctures
- you have a history of problems with your spine or have had surgery on your spine.
If you take Savaysa and receive spinal anesthesia or have a spinal puncture, your doctor should watch you closely for symptoms of spinal or epidural blood clots. Tell your doctor right away if you have back pain, tingling, numbness (especially in your legs and feet), muscle weakness, loss of control of the bowels or bladder (incontinence).
- Savaysa is not for people with mechanical heart valves or people who have moderate to severe narrowing (stenosis) of their mitral valve.
- Savaysa is not for use in people with antiphospholipid syndrome (APS), especially with positive triple antibody testing, who have a history of blood clots.
See “What are the possible side effects of Savaysa?” for more information about side effects.
Who should not take Savaysa?
Do not take Savaysa if you currently have certain types of abnormal bleeding.
What should I tell my healthcare provider before taking Savaysa?
Before taking Savaysa, tell your doctor about all of your medical conditions, including if you:
- have liver or kidney problems
- have antiphospholipid syndrome
- have ever had bleeding problems
- have a mechanical heart valve
- have cancer of the stomach or intestine (gastrointestinal cancer)
- are pregnant or plan to become pregnant. It is not known if Savaysa will harm your unborn baby. Tell your doctor right away if you become pregnant during treatment with Savaysa.
- are breastfeeding or plan to breastfeed. It is not known if Savaysa passes into your breast milk. Do not breastfeed during treatment with Savaysa.
Tell all of your doctors and dentists that you are taking Savaysa. They should talk to the doctor who prescribed Savaysa for you, before you have any surgery, medical or dental procedure.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some of your other medicines may affect the way Savaysa works. Certain medicines may increase your risk of bleeding or stroke when taken with Savaysa. See “What is the most important information I should know about Savaysa?”
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How to take Savaysa?
- Take Savaysa exactly as prescribed by your doctor.
- Your doctor will decide how long you should take Savaysa. Do not change your dose or stop taking Savaysa unless your doctor tells you to. If you are taking Savaysa for nonvalvular atrial fibrillation, stopping Savaysa may increase your risk of having a stroke.
- Take Savaysa with or without food.
- If you have difficulty swallowing the tablet whole, talk to your doctor about other ways to take Savaysa.
- If you miss a dose of Savaysa, take it as soon as you remember on the same day. Take your next dose at your usual time the next day. Do not take more than one dose of Savaysa at the same time to make up for a missed dose.
- Do not run out of Savaysa. Refill your prescription before you run out. If you take too much Savaysa, go to the nearest hospital emergency room or call your doctor right away.
- Call your doctor right away if you fall or injure yourself, especially if you hit your head. Your doctor may need to check you.
What are side effects of Savaysa?
Savaysa can cause serious side effects.
See “What is the most important information I should know about Savaysa?”
The most common side effects in people who take Savaysa for nonvalvular atrial fibrillation include bleeding and low red blood cell count (anemia).
The most common side effects in people who take Savaysa for deep vein thrombosis and pulmonary embolism include, bleeding, rash, abnormal liver function tests and low red blood cell count (anemia).
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Label
PRINCIPAL DISPLAY PANEL
NDC 65597-201-30
Savaysa
(edoxaban) tablets
15 mg
30 TABLETS
Rx Only

PRINCIPAL DISPLAY PANEL
NDC 65597-202-30
Savaysa
(edoxaban) tablets
30 mg
30 TABLETS
Rx Only
PRINCIPAL DISPLAY PANEL
NDC 65597-202-05
Savaysa
(edoxaban) tablets
30 mg
Package contains 5 blister packs of 10 tablets each
Rx Only
General information about the safe and effective use of Savaysa
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Savaysa for a condition for which it was not prescribed. Do not give Savaysa to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or doctor for information about Savaysa that is written for health professionals.
How supplied/storage
SAVAYSA (edoxaban) is supplied as round shaped, film-coated, non-scored tablets containing edoxaban tosylate equivalent to 60, 30 or 15 mg of SAVAYSA, packaged in bottles and blisters.
| NDC 65597-xxx-yy | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strength | Color | Deboss | yy | |||||
| xxx | Bottle of | Blister of | ||||||
| 30 | 90 | 500 | 10 × 10* | 10 × 5† | ||||
|
||||||||
| 15 mg | orange | DSC L15 | 201 | 30 | – | – | – | – |
| 30 mg | pink | DSC L30 | 202 | 30 | 90 | 50 | 10 | 05 |
| 60 mg | yellow | DSC L60 | 203 | 30 | 90 | 50 | 10 | 05 |
Store at 20-25°C (68-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
Keep out of the reach of children.
What are the ingredients in Savaysa?
Active ingredient: edoxaban tosylate monohydrate
Inactive ingredients: mannitol, pregelatinized starch, crospovidone, hydroxypropyl cellulose, magnesium stearate, talc, and carnauba wax. The color coatings contain hypromellose, titanium dioxide, talc, polyethylene glycol 8000, iron oxide yellow (60 mg tablets and 15 mg tablets), and iron oxide red (30 mg tablets and 15 mg tablets).
SRC: NLM .


