Repatha
Drug class: PCSK9 inhibitors
What is Repatha?
Repatha is an injectable prescription medicine used in adults with cardiovascular disease to reduce the risk of heart attack, stroke, and certain types of heart surgery, along with diet alone or together with other cholesterol-lowering medicines in adults with high blood cholesterol levels called primary hyperlipidemia (including a type of high cholesterol called heterozygous familial hypercholesterolemia) to reduce low density lipoprotein (LDL) or bad cholesterol. along with diet and other LDL-lowering medicine in people with a type of high cholesterol called homozygous familial hypercholesterolemia (HoFH), who need additional lowering of LDL cholesterol.
It is not known if Repatha is safe and effective in children with HoFH who are younger than 13 years of age or in children who do not have HoFH.
Description
Evolocumab is a human monoclonal immunoglobulin G2 (IgG2) directed against human proprotein convertase subtilisin kexin type 9 (PCSK9). Evolocumab has an approximate molecular weight (MW) of 144 kDa and is produced in genetically engineered mammalian (Chinese hamster ovary) cells.
REPATHA is a sterile, preservative-free, clear to opalescent, colorless to pale yellow solution for subcutaneous use. Each 1 mL single-dose prefilled syringe and single-dose prefilled SureClick® autoinjector contains 140 mg evolocumab, acetate (1.2 mg), polysorbate 80 (0.1 mg), proline (25 mg) in Water for Injection, USP. Sodium hydroxide may be used to adjust to a pH of 5.0. Each single-dose Pushtronex® system (on-body infusor with prefilled cartridge) delivers a 3.5 mL solution containing 420 mg evolocumab, acetate (4.2 mg), polysorbate 80 (0.35 mg), proline (89 mg) in Water for Injection, USP. Sodium hydroxide may be used to adjust to a pH of 5.0.
Mechanism of Action
Evolocumab is a human monoclonal IgG2 directed against human proprotein convertase subtilisin kexin type 9 (PCSK9). PCSK9 binds to the low-density lipoprotein receptor (LDLR) on the surface of hepatocytes to promote LDLR degradation within the liver. By inhibiting the binding of PCSK9 to LDLR, evolocumab increases the number of LDLRs available to clear LDL from the blood, thereby lowering LDL-C levels.
Who should not use Repatha?
Do not use Repatha if you are allergic to evolocumab or to any of the ingredients in Repatha. See the end of this guide for a complete list of ingredients in Repatha.
What should I tell my healthcare provider before using Repatha?
Before you start using Repatha, tell your healthcare provider about all your medical conditions, including if you:
- are allergic to rubber or latex. The needle covers on the single-use prefilled syringes and within the needle caps on the single-use prefilled SureClick autoinjectors contain dry natural rubber. The single-use Pushtronex system (on-body infusor with prefilled cartridge) is not made with natural rubber latex.
- are pregnant or plan to become pregnant. It is not known if Repatha will harm your unborn baby. Tell your healthcare provider if you become pregnant while taking Repatha.
Pregnancy Registry. There is a pregnancy registry for women who take Repatha during pregnancy. The purpose of this registry is to collect information about your health and your baby’s health. You can talk to your healthcare provider or contact 1-877-311-8972 or go to https://mothertobaby.org/ongoing-study/Repatha/ to enroll in this registry or get more information.
- are breastfeeding or plan to breastfeed. You and your healthcare provider should decide if you will take Repatha or breastfeed.
Tell your healthcare provider or pharmacist about any prescription and over-the-counter medicines, vitamins, or herbal supplements you take.
How should I use Repatha?
- See the detailed “Instructions for Use” that come with your medication about the right way to prepare and give Repatha.
- Use Repatha exactly as your healthcare provider tells you to use it.
- Repatha is given under the skin (subcutaneously), every 2 weeks or 1 time each month.
- Repatha comes as a single-use (1 time) prefilled autoinjector (SureClick autoinjector), as a single-use prefilled syringe or as a single-use Pushtronex system (on-body infusor with prefilled cartridge). Your healthcare provider will prescribe the type and dose that is best for you.
- If your healthcare provider prescribes you the monthly dose, you may use:
- a single-use on-body infusor with prefilled cartridge to give the injection over 9 minutes, or
- 3 separate injections in a row, using a different single-use prefilled syringe or single-use prefilled autoinjector for each injection. Give all of these injections within 30 minutes.
- If your healthcare provider decides that you or a caregiver can give Repatha, you or your caregiver should receive training on the right way to prepare and inject Repatha. Do not try to inject Repatha until you have been shown the right way by your healthcare provider or nurse.
- If you are using the prefilled autoinjector, put the yellow safety guard (needle inside) of the SureClick autoinjector on the skin before injecting.
- Do not inject Repatha together with other injectable medicines at the same injection site.
- Always check the label of your single-use prefilled autoinjector, single-use prefilled syringe, or single-use on-body infusor with prefilled cartridge to make sure you have the correct medicine and the correct dose of Repatha before each injection.
- If you forget to use Repatha or are not able to take the dose at the regular time, inject your missed dose as soon as you remember, as long as it is within 7 days of the missed dose.
- If it is more than 7 days from the missed dose and you are using the every-2-week dose, inject the next dose based on your original schedule. This will put you back on your original schedule.
- If it is more than 7 days from the missed dose and you are using the 1 time each-month dose, inject the dose and start a new schedule using this date.
If you are not sure when to take Repatha after a missed dose, ask your healthcare provider or pharmacist.
- If your healthcare provider has prescribed Repatha along with other cholesterol-lowering medicines, follow instructions from your healthcare provider. Read the patient information for those medicines.
- If you use more Repatha than you should, talk to your healthcare provider or pharmacist.
- Do not stop using Repatha without talking with your healthcare provider. If you stop using Repatha, your cholesterol levels can increase.
What are possible side effects of Repatha?
Repatha can cause serious side effects including:
- Serious allergic reactions. Some people taking Repatha have had serious allergic reactions. Stop taking Repatha and call your healthcare provider or seek emergency medical help right away if you have any of these symptoms:
- trouble breathing or swallowing
- raised bumps (hives)
- rash, or itching
- swelling of the face, lips, tongue, throat or arms
The most common side effects of Repatha include:
- runny nose
- sore throat
- symptoms of the common cold, flu or flu-like symptoms
- back pain
- high blood sugar levels (diabetes)
- redness, pain, or bruising at the injection site
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Repatha. Ask your healthcare provider or pharmacist for more information.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Repatha.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Repatha for a condition for which it was not prescribed. Do not give Repatha to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about Repatha that is written for healthcare professionals.
How should I store Repatha?
- Store refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton.
- Alternatively, Repatha can be kept at room temperature at 68°F to 77°F (20°C to 25°C) in the original carton; however, under these conditions, Repatha must be used within 30 days. If not used within the 30 days, discard Repatha.
- Protect Repatha from direct light and do not expose to temperatures above 25°C (77°F).
- Keep out of sight and reach of children.
What are the ingredients in Repatha?
- Active Ingredient: evolocumab
- Inactive Ingredients: proline, glacial acetic acid, polysorbate 80, water for injection, and sodium hydroxide.
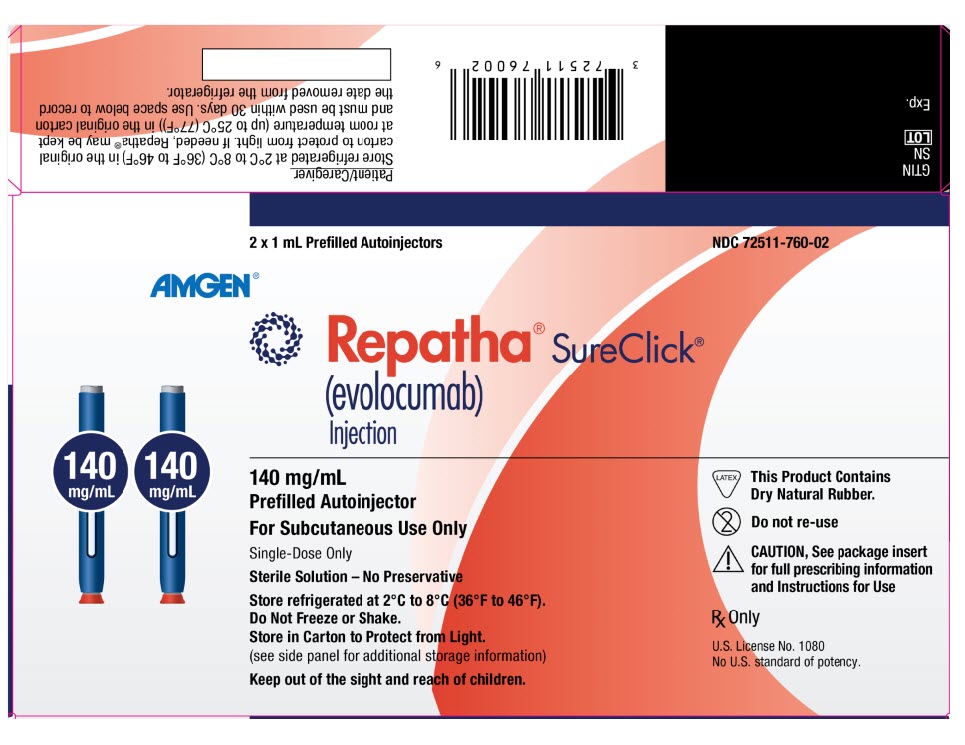
Label
PRINCIPAL DISPLAY PANEL
- 1 x 1 mL Prefilled Syringe
NDC 55513-750-01
AMGEN®
Repatha®
(evolocumab)
Injection
140 mg/mL
140 mg/mL
Prefilled Syringe
For Subcutaneous Use Only
PRINCIPAL DISPLAY PANEL
- 1 x 3.5 mL Prefilled Cartridge
1 On-Body Infusor
NDC 55513-770-01
AMGEN®
Repatha® Pushtronex® system
(evolocumab)
On-Body Infusor and Prefilled Cartridge
420 mg/3.5 mL
Shorter Injection Time
420 mg/3.5 mL
For Subcutaneous Use Only

SRC: NLM .
