Rebif
Generic name: interferon beta-1a
Drug class: Interferons
Medically reviewed by A Ras MD.
What is Rebif?
Rebif is a prescription medicine used to treat relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. It is a form of protein called beta interferon that is produced in the body.
It is not known if Rebif is safe and effective in children.
Description
REBIF (interferon beta-1a) is a purified 166 amino acid glycoprotein with a molecular weight of approximately 22,500 daltons. It is produced by recombinant DNA technology using genetically engineered Chinese Hamster Ovary cells into which the human interferon beta gene has been introduced. The amino acid sequence of REBIF is identical to that of natural fibroblast derived human interferon beta. Natural interferon beta and interferon beta-1a (REBIF) are glycosylated with each containing a single N-linked complex carbohydrate moiety.
Using a reference standard calibrated against the World Health Organization natural interferon beta standard (Second International Standard for Interferon, Human Fibroblast GB 23 902 531), REBIF has a specific activity of approximately 270 million international units (MIU) of antiviral activity per mg of interferon beta-1a determined specifically by an in vitro cytopathic effect bioassay using WISH cells and Vesicular Stomatitis virus. REBIF 8.8 mcg, 22 mcg and 44 mcg contains approximately 2.4 million international units, 6 million international units or 12 million international units, respectively, of antiviral activity using this method.
REBIF (interferon beta-1a) is formulated as a sterile solution in a prefilled syringe or REBIF Rebidose autoinjector intended for subcutaneous (sc) injection. Each 0.5 mL (0.5 cc) of REBIF contains either 22 mcg or 44 mcg of interferon beta-1a, 2 mg or 4 mg albumin (human), 27.3 mg mannitol, 0.4 mg sodium acetate, and water for injection. Each 0.2 mL (0.2 cc) of REBIF contains 8.8 mcg of interferon beta-1a, 0.8 mg albumin (human), 10.9 mg mannitol, 0.16 mg sodium acetate, and water for injection.
Mechanism of Action
The mechanism(s) by which REBIF (interferon beta-1a) exerts its therapeutic effects in patients with multiple sclerosis is unknown.
What is the most important information I should know about Rebif?
Rebif can cause serious side effects. Tell your healthcare provider right away if you have any of the symptoms listed below while taking Rebif.
1. Behavioral health problems including depression and suicidal thoughts. You may have mood problems including:
- depression (feeling hopeless or feeling bad about yourself)
- thoughts of hurting yourself or suicide
2. Liver problems or worsening of liver problems including liver failure. Symptoms may include:
- nausea
- loss of appetite
- tiredness
- dark colored urine and pale stools
- yellowing of your skin or the white part of your eye
- bleeding more easily than normal
- confusion
- sleepinessDuring your treatment with Rebif you will need to see your healthcare provider regularly and have regular blood tests to check for side effects.
3. Serious allergic and skin reactions. Symptoms may include:
- itching
- swelling of your face, eyes, lips, tongue or throat
- trouble breathing
- anxiousness
- feeling faint
- skin rash, hives, sores in your mouth, or skin blisters and peels
4. Injection site problems. Symptoms at the injection site may include:
- redness
- pain
- swelling
- color changes (blue or black)
- drainage of fluid
Who should not take Rebif?
Do not take Rebif if you:
- are allergic to interferon beta, human albumin, or any of the ingredients in Rebif. See the end of this Medication Guide for a complete list of ingredients in Rebif.
What should I tell my healthcare provider before taking Rebif?
Before you take Rebif, tell your healthcare provider if have or have had any of the following conditions:
- mental illness, including depression and suicidal behavior
- liver problems
- bleeding problems or blood clots
- low blood cell counts
- seizures (epilepsy)
- thyroid problems
- drink alcohol
- you are pregnant or plan to become pregnant. It is not known if Rebif will harm your unborn baby.
- you are breastfeeding or plan to breastfeed. Rebif may pass into your breast milk. Talk with your healthcare provider about the best way to feed your baby if you take Rebif.
Tell your healthcare provider about all medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
Rebif and other medicines may affect each other causing side effects.
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use Rebif?
- See the Instructions for Use that come with Rebif on how to prepare and give an injection of Rebif using a prefilled syringe. For the Rebif Rebidose autoinjector, read the Instructions for Use that comes with the Rebif Rebidose autoinjector.
- Your healthcare provider should show you how to prepare and measure your dose of Rebif and how to inject your Rebif before you use it for the first time.
- Rebif is given by injection under the skin (subcutaneous injection) on the same 3 days a week, for example, Monday, Wednesday and Friday.
- Your injections should be at least 48 hours apart. Take them the same time each day.
- Inject Rebif exactly as your healthcare provider tells you.
- Your healthcare provider will tell you how much Rebif to inject, and may change the dose based on how your body responds. Do not inject more than your healthcare provider tells you to.
- Do not change your dose unless your healthcare provider tells you to.
- Change (rotate) your injection site you choose with each injection. This will help decrease the chance that you will have an injection site reaction.
- Do not inject Rebif into an area of the body where the skin is irritated, reddened, bruised, infected or scarred in any way.
- Rebif comes as a:
- prefilled syringe (Rebif)
- single-use prefilled autoinjector (Rebif Rebidose autoinjector)Your healthcare provider will decide which is best for you. Always use a new, unopened, prefilled syringe of Rebif or Rebif Rebidose autoinjector for each injection. Do not reuse prefilled syringes or Rebif Rebidose autoinjectors.
What are the possible side effects of Rebif?
Rebif may cause serious side effects, including:
- See “What is the most important information I should know about Rebif?”
- Blood problems. Rebif can affect your bone marrow and cause low red and white blood cell, and platelet counts. In some people, these blood cell counts may fall to dangerously low levels. If your blood cell counts become very low, you can get infections and problems with bleeding and bruising. Your healthcare provider may ask you to have regular blood tests to check for blood problems.
- Seizures. Some people have had seizures while taking Rebif.
The most common side effects of Rebif include:
- flu-like symptoms. You may have flu-like symptoms when you first start taking Rebif. You may be able to manage these flu-like symptoms by taking over-the-counter pain and fever reducers. For many people, these symptoms lessen or go away over time. Symptoms may include:
- muscle aches
- nausea
- fever
- tiredness
- chills
- stomach pain
- change in liver blood tests
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Rebif. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
General information about the safe and effective use of Rebif
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Rebif for a condition for which it was not prescribed. Do not give Rebif to other people, even if they have they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Rebif. If you would like more information, talk with your healthcare provider. You may ask your healthcare provider or pharmacist for information about Rebif that is written for healthcare professionals.
For more information, go to www.REBIF.com or call toll-free 1-877-447-3243.
How should I store Rebif?
- Store Rebif in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze Rebif.
- If you cannot refrigerate your Rebif, you can store your Rebif at temperatures above 36°F and below 77°˚F (2°C to 25°C) for up to 30 days.
- Keep Rebif away from heat and light.
Keep Rebif and all medicines out of the reach of children.
What are the ingredients in Rebif?
Active ingredient: interferon beta-1a
Inactive ingredients: albumin (human), mannitol, sodium acetate, water for injection
Instructions for use
Rebif instructions of use
Important: For the Rebif Rebidose autoinjector, read the Instructions for Use that come with the Rebif Rebidose autoinjector below.
Parts of your Rebif Prefilled Syringe (See FIGURE A).
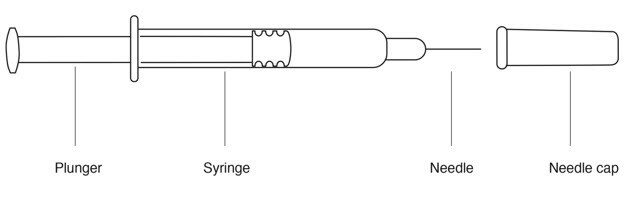
Figure A
Supplies needed for a Rebif Injection (See FIGURE B):
- Rebif prefilled syringe
- alcohol pad or cotton balls and rubbing alcohol
- small adhesive bandage strip if desired
- puncture resistant safety container for disposal of used syringes. See “Disposing of your needles and syringes section” in Step 4 of the IFU.
- antibacterial soap
- an over-the-counter pain or fever reducing medicine, if your healthcare provider has recommended that you take this before, at the same time, or after you give yourself Rebif to help decrease the fever, chills, sweating and muscle aches (flu-like symptoms) that may happen.
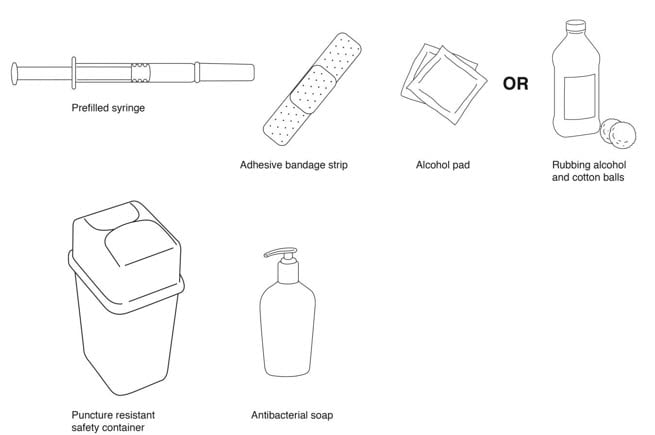
Figure B
Titration (Dosing) Schedule
- When first starting treatment with Rebif, your healthcare provider may prescribe either the 22 mcg or 44 mcg dose of Rebif. You should gradually increase the dose over 4 weeks, starting at 20% of the prescribed dose for the first 2 weeks, half-dose for the second 2 weeks (weeks 3 and 4), and then the full dose prescribed by your healthcare provider.
If your prescribed dose is 22 mcg of Rebif, a Rebif Titration Pack containing 6 prefilled syringes with 8.8 mcg and 6 prefilled syringes with 22 mcg should be prescribed to you for use during the 4-week starting period. Table 1 explains the amount to inject using the Rebif Titration Pack syringes to gradually increase to 22 mcg.
Table 1: Titration Schedule for a 22 mcg Prescribed Dose*
| Week of Use | Syringe to Use | Amount of syringe |
| Week 1 Titration | 8.8 mcg syringe | Use half of syringe |
| Week 2 Titration | 8.8 mcg syringe | Use half of syringe |
| Week 3 Titration | 22 mcg syringe | Use half of syringe |
| Week 4 Titration | 22 mcg syringe | Use half of syringe |
| Week 5 and on | 22 mcg syringe or autoinjector | Use full syringe or autoinjector |
*Only prefilled syringes can be used to titrate to the 22 mcg Prescribed Dose
If your prescribed dose is 44 mcg, you may be prescribed either a Rebif Titration Pack (described above) or Rebif Rebidose Titration Pack containing 6 autoinjectors with 8.8 mcg and 6 autoinjectors with 22 mcg for use during the 4 week titration period. Table 2 explains the amount to inject using the Rebif Titration Pack or Rebif Rebidose Titration Pack to gradually increase to 44 mcg.
Table 2: Titration Schedule for a 44 mcg Prescribed Dose*
| Week of Use | Syringe to Use | Amount of syringe |
| Week 1 Titration | 8.8 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 2 Titration | 8.8 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 3 Titration | 22 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 4 Titration | 22 mcg syringe or autoinjector | Use full syringe or autoinjector |
| Week 5 and on | 44 mcg syringe or autoinjector | Use full syringe or autoinjector |
*Prefilled syringes or autoinjectors can be used to titrate to 44 mcg Prescribed Dose
Step 1. Preparing for your Rebif Injection
- Check the expiration date. Do not use if the medication is expired. The expiration date is printed on the syringe, plastic syringe packaging and carton.
- Remove your Rebif syringe from the refrigerator at least 30 minutes before you plan to use it so it can warm to room temperature. Do not heat or microwave the medication.
- Be sure that the dose, either, 8.8 mcg, 22 mcg or 44 mcg, described on the carton is the same as the dose prescribed by your healthcare provider.
- Remove the Rebif syringe from the plastic packaging. Keep the needle capped.
- Look at the contents of the syringe carefully. The liquid should be clear to slightly yellow. Do not use if the liquid is cloudy, discolored or contains particles. Use a different syringe.
Step 2. Choose and Prepare your Injection Site
- The best sites for giving yourself an injection are those areas with a layer of fat between the skin and muscle, like your thigh, the outer surface of your upper arm, your stomach or buttocks.
- Do not use the area near your waistline or within 2 inches of your navel. If you are very thin, use only the thigh or outer surface of the arm for injection.
- Use a different site each time you inject such as the thigh, hip, stomach or upper arm (See FIGURE C).
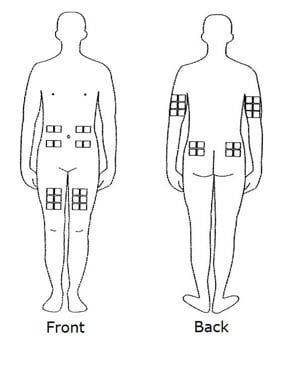
Figure C
- Do not inject Rebif into an area of your body where the skin is irritated, reddened, bruised, infected or abnormal in any way.
- Wash your hands thoroughly with antibacterial soap before preparing to inject the medicine.
- Clean the injection site with an alcohol pad or cotton ball with rubbing alcohol using a circular motion. To avoid stinging, you should let your skin dry before you inject Rebif.
Step 3. Inject your Rebif
- Remove the needle cap from the syringe needle.
- If your healthcare provider has told you to use less than the full 0.5 mL dose, slowly push the plunger in until the amount of medicine left in the syringe is the amount healthcare provider told you to use.
- Use your thumb and forefinger to pinch a pad of skin surrounding the cleaned injection site (See FIGURE D). Hold the syringe like a pencil with your other hand.
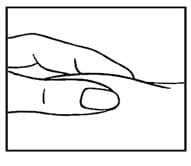
Figure D
- While still pinching the skin, quickly insert the needle like a dart at about a 90 degree angle (just under the skin) into the pad of tissue as shown (See FIGURE E).
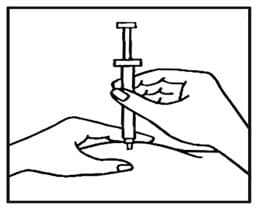
Figure E
- After the needle is in, remove the hand that you used to pinch your skin and inject the medicine using a slow, steady push on the plunger until all the medicine is injected and the syringe is empty (See FIGURE F).
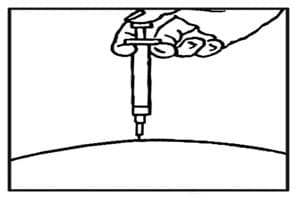
Figure F
- Withdraw the needle and apply gentle pressure to the injection site with a dry cotton ball or sterile gauze. Applying a cold compress or ice pack to the injection site after injection may help reduce local skin reactions.
- Put a small adhesive bandage strip over the injection site, if desired.
- Keep a record of the date and location of each injection.
- After 2 hours, check the injection site for redness, swelling, or tenderness. If you have a skin reaction and it does not clear up in a few days, call your healthcare provider.
Step 4. Disposing of your Needles and Syringes
- Put your used needles, syringes, and autoinjectors, including Rebif, in an FDA-cleared sharps container right away after use. Do not throw away (dispose of) any syringes or autoinjectors in your household trash.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:- made of a heavy-duty plastic,
- closed with a tight-fitting, puncture-resistant lid,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used autoinjectors and syringe needles. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Instructions for use last revised 11/2015
Rebif Rebidose autoinjector instructions for use
Exclusively for use with
Rebif (interferon beta-1a) subcutaneous injection
Please read these instructions carefully before you start using the Rebif Rebidose and remember, you must have received the appropriate training before injecting.
If at any time, you have questions or concerns, please contact your health care provider or call MS LifeLines at 1-877-447-3243.
Rx Only
| Contents |
| Rebif Rebidose features |
 Warnings Warnings |
| Important information |
| How Supplied |
| Titration |
| Choosing an injections site |
Step 1: Prepare for your injection
|
| Step 2: Administer your injection |
| Step 3: Finish your injection |
| Disposal |
| Storage |
| Travel |
| Notes |
Rebif Rebidose features
Before injection: After injection:
 Warnings
Warnings
- Always use the injection technique advised by your health care provider and contact them with any questions you might have.
- Always keep the Rebidose autoinjector out of the reach of children.
- Do not share the Rebidose with anyone. Doing so may result in injury, including the transmission of infectious blood-borne diseases. Adhere to strict safety and antiseptic precautions at all times.
- Do not insert your fingers into the opening of the safety guard.
- Do not try to re-use a Rebidose single-use autoinjector that has already been used.
- Adhere to proper disposal procedures to avoid a needle stick injury.
- Check the expiration date on the Rebidose autoinjector label and its carton. If the medication has expired, Do not use it.
- Examine the contents of the syringe carefully through the transparent housing. The liquid should be clear or slightly yellow. Inspect for cracks or breakage in the syringe or other parts. Do not use Rebidose if it is cracked or broken or if the liquid is cloudy, discolored or contains particles.
- If the Rebidose has been dropped more than 3 feet, or if it looks damaged for any reason, Do not use it. Instead, dispose of it in an acceptable biohazard (sharps) container and use a new Rebidose autoinjector.
- Contact your health care provider about any damaged Rebidose autoinjector.
Important Information
- Store your Rebidose autoinjectors in a refrigerator until ready for use.
- If you or someone around you is injured by the needle, contact your health care provider immediately and dispose of the Rebidose in an acceptable biohazard (sharps) container.
- Injecting Rebif the medicine in the Rebidose autoinjector may cause skin reactions including soreness, redness, pain, bruising, or swelling. Tell your health care provider about any skin reactions that become swollen, painful, or look infected and do not heal within a few days.
- Use a different site each time you inject. Do not inject Rebif where your skin is irritated, reddened, bruised, infected or abnormal in any way.
- The Rebidose autoinjector contains a glass syringe. In very rare cases, the syringe may break during the injection. If this happens, immediately dispose of any broken glass and the damaged Rebidose autoinjector in the biohazard (sharps) container, taking necessary precautions to avoid injury. Contact your health care provider or MS LifeLines at 1-877-447-3243 immediately.
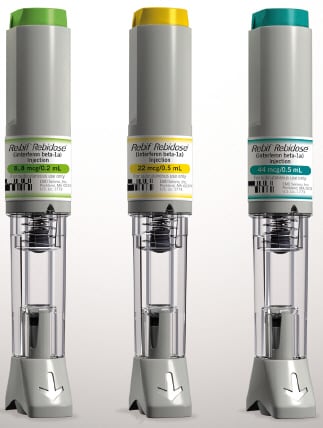
How Supplied
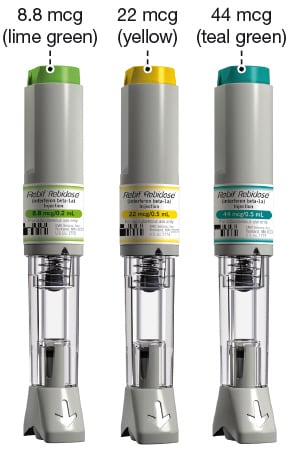
Rebidose is available in 3 dose sizes. Check that the dose of Rebif is the one prescribed for you by looking at the Rebidose carton and at the dose label on the Rebidose autoinjector.
Rebidose autoinjectors are supplied in these 3 packaging presentations:
- Titration Pack
- 6 Rebif 8.8 mcg Rebidose autoinjectors with a lime green injector button.
- 6 Rebif 22 mcg Rebidose autoinjectors with a yellow injector button.
- 12 Rebif 22 mcg Rebidose autoinjectors with a yellow injector button.
- 12 Rebif 44 mcg Rebidose autoinjectors with a teal green injector button.
Titration
At the beginning of Rebif treatment, your health care provider may prescribe a titration phase to gradually increase to the intended dosage.
The Rebif Titration Pack contains six 8.8 mcg Rebidose autoinjectors with a lime green injector button and six 22 mcg Rebidose autoinjectors with a yellow injector button.
The titration period lasts 4 weeks and during this time, you will administer 12 injections:
- Weeks 1 and 2: Use one 8.8 mcg autoinjector three times a week, at least 48 hours apart.
- Weeks 3 and 4: Use one 22 mcg autoinjector three times a week, at least 48 hours apart.
Your health care provider may provide a different dose and schedule.
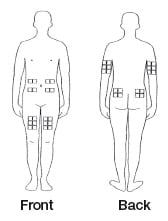
Choosing an injection site
The best sites for giving a subcutaneous injection are those areas with a layer of fat between the skin and muscle. These include the thigh, the outer surface of the upper arm, the stomach and the buttocks. Do not use the area near your waistline or within 2 inches of your navel.
Only choose one site for injection. Use a different site each time you inject.
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
 Caution
Caution
Do not inject Rebif where your skin is irritated, reddened, bruised, infected or abnormal in any way.
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
Step 1: Prepare for your injection
1.1) Before you begin, you may find injecting more comfortable if you allow time for the Rebif to warm up to room temperature before injecting. Therefore, it is recommended that you remove the Rebidose autoinjector from the refrigerator at least 30 minutes prior to use.
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
 Caution
Caution
Do not heat or microwave a Rebidose autoinjector.
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
1.2) Put all of the items listed below within easy reach on a table or other stable surface:
- 1 Rebidose autoinjector containing Rebif
- Alcohol wipes/swabs or cotton balls and rubbing alcohol
- Small adhesive bandage strip (if needed)
- A biohazard (sharps) container (see DISPOSAL section on page 22)
1.3) Wash your hands thoroughly with antibacterial soap.
1.4) Open the tray over a table or soft surface by peeling back the plastic covering and remove 1 Rebidose autoinjector. Do not remove the needle cap until you are ready to inject because the autoinjector could roll off the table and become unsterile.
1.5) Check the expiration date on the Rebidose autoinjector label and its carton. If the medication has expired, do not use it.
1.6) Examine the contents of the syringe carefully through the transparent housing. The liquid should be clear or slightly yellow.
1.7) Inspect for cracks or breakage in the syringe or other parts. Do not use Rebidose if it is cracked or broken or if the liquid is cloudy, discolored or contains particles.
1.8) Use an alcohol wipe to clean the injection site. Let your skin dry to prevent stinging during injection.
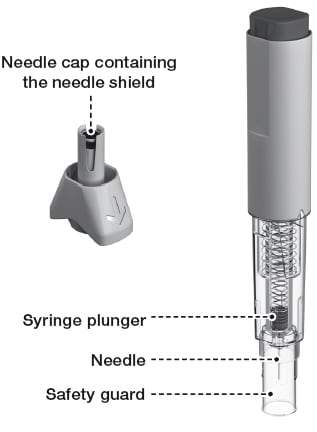
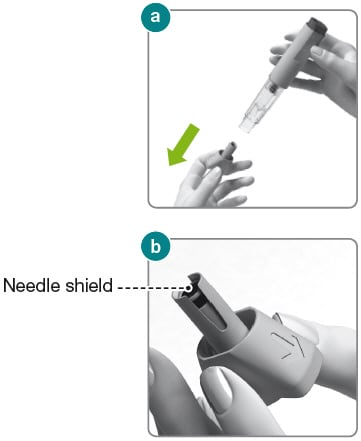
1.9) Hold the Rebidose by the injector body (fig a ) and pull off the needle cap.
1.10) Look inside the needle cap to make sure you see a black needle shield inside the cap (fig b ).
1.11) Dispose of the needle cap and proceed to the injection step without delay.
 Warning Warning
If the Rebidose has been dropped more than 3 feet, or if it looks damaged for any reason, do not use it. Instead, dispose of it in an acceptable biohazard (sharps) container and use a new Rebidose autoinjector. |
Step 2: Administer your injection
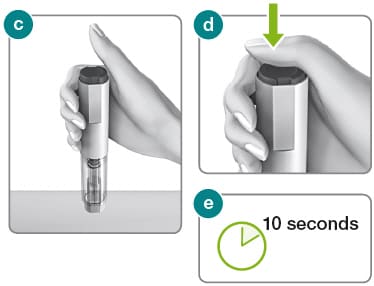
2.1) Hold the Rebidose autoinjector in your palm with your thumb above the injector button.
2.2) Place the Rebidose upright with the needle end flat against your skin at a 90° angle. Push the Rebidose against your skin until you feel resistance; this action unlocks the injector button (fig c ).
2.3) Keep the Rebidose pressed firmly against the skin and use your thumb to push the injector button (fig d ). You will hear a click, which means the injection has begun.
2.4) Keep the Rebidose pressed firmly against your skin for at least 10 seconds while the medication is dispensed (fig e ).
If you have any problems, contact MS LifeLines at 1-877-447-3243.
 Caution
Caution
If the injection does not start, release the injector button and make sure that the Rebidose is still pressed firmly against your skin. Then push firmly on the injector button, listening for the click that indicates the start of the injection.
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
Step 3: Finish your injection
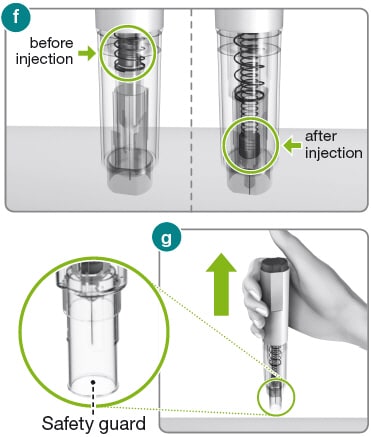
3.1) Before lifting the Rebidose from your skin, make sure that the syringe plunger has moved to the bottom and that the entire dose has been injected (fig f ).
3.2) Lift the Rebidose from the injection site. The safety guard slides down and locks into place to protect you from the needle (fig g ). If any liquid is left in the syringe or if you have any other problems, contact your health care provider or MS LifeLines at 1-877-447-3243.
Do not try to put the needle cap on the used Rebidose autoinjector. It is no longer needed.
3.3) If desired, use a small adhesive bandage to cover the injection site.
Disposal
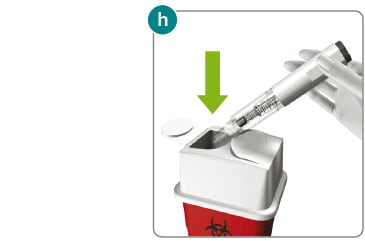
1) Put your used Rebidose autoinjectors in a FDA-cleared sharps disposal container right away after use (fig h ). Do not throw them away (dispose of) in your household trash.
2) If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic;
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out;
- upright and stable during use;
- leak-resistant; and
- properly labeled to warn of hazardous waste inside the container.
3) When your sharps disposal container is almost full, you will need to follow your community guidelines for the correct way to dispose of it. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal
4) Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
 Caution
Caution
Always keep the biohazard (sharps) container out of the sight and reach of children.
– – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – – –
 Warning Warning
|
Storage
- Each Rebidose autoinjector contains a single dose of Rebif.
- Store Rebidose autoinjectors in the refrigerator between 36°F and 46°F (2°C to 8°C). Do not freeze. If a refrigerator is not available, Rebidose may be stored between 36˚F and 77˚F (2˚C to 25˚C) for up to 30 days away from heat and light.
- Do not put them in or near the freezer compartment. Do not inject Rebif that you know or suspect has been frozen.
- Keep the Rebidose in its packaging and only open one when you need it. Refer to the Rebif drug package insert and medication guide for specific storage guidelines.
Travel
- When you travel, take 3 Rebidose autoinjectors with you for each week of your trip. Take some extra in case you are away for longer than expected.
- Remember to pack an empty biohazard (sharps) container for proper disposal.
- When traveling by air, always carry Rebidose autoinjectors in your hand luggage because the aircraft luggage compartment can be very cold and the Rebif could freeze.
- It is safe to pass Rebidose autoinjectors through x-ray machines, but you may need a note from your health care provider to allow you to carry them aboard an airplane.
- Prior to traveling, always check with your airline and your health care provider about bringing injectable medication with you.
Notes
You may find it helpful to record information about your 3 weekly injections (Inj.) to discuss with your health care provider. Including the details about the injection site, injection date and time and whether or not you had a site reaction.
Label
PRINCIPAL DISPLAY PANEL – KIT CARTON – 0188
- Rebif® Rebidose®
(interferon beta-1a)
Injection - NDC 44087-0188-1
- 6 single-use 8.8 mcg / 0.2 mL autoinjectors
6 single-use 22 mcg / 0.5 mL autoinjectors - Rx only
- 8.8 mcg / 0.2 mL
22 mcg / 0.5 mL - Titration Pack
For subcutaneous injection - Attention pharmacist:
Each patient is required to receive
the enclosed Medication Guide - EMD
Serono
PRINCIPAL DISPLAY PANEL – 12 SYRINGE CARTON – 0022
- Rebif® 22 mcg/0.5 mL
(interferon beta-1a) - NDC 44087-0022-3
- For subcutaneous injection
Rx only
Medication Guide for patients enclosed - 12 Single-use prefilled syringes
- EMD
Serono
SRC: NLM .
