Qulipta
Generic name: atogepant
Dosage form: tablets
Drug class: CGRP inhibitors
Medically reviewed by A Ras MD.
What is Qulipta?
Qulipta is a prescription medicine used for the preventive treatment of episodic migraine in adults.
It is not known if Qulipta is safe and effective in children.
Description
The active ingredient of QULIPTA is atogepant, a calcitonin gene-related peptide (CGRP) receptor antagonist. The chemical name of atogepant is (3′S)-N-[(3S,5S,6R)-6-methyl-2-oxo-1-(2,2,2-trifluoroethyl)-5-(2,3,6-trifluorophenyl)piperidin-3-yl]-2′-oxo-1′,2′,5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3′-pyrrolo[2,3-b]pyridine]-3-carboxamide, and it has the following structural formula:
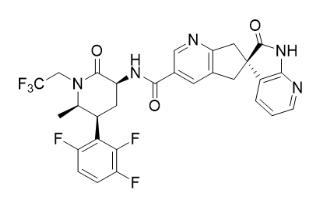
The molecular formula is C29H23F6N5O3 and molecular weight is 603.5. Atogepant is a white to off-white powder. It is freely soluble in ethanol, soluble in methanol, sparingly soluble in acetone, slightly soluble in acetonitrile, and practically insoluble in water.
QULIPTA is available as tablets for oral administration containing 10 mg, 30 mg, or 60 mg atogepant. The inactive ingredients include colloidal silicon dioxide, croscarmellose sodium, mannitol, microcrystalline cellulose, polyvinylpyrrolidone vinyl acetate copolymer, sodium chloride, sodium stearyl fumarate, and vitamin E polyethylene glycol succinate.
Mechanism of Action
Atogepant is a calcitonin gene-related peptide (CGRP) receptor antagonist.
What should I tell my healthcare provider before taking Qulipta?
Before you take Qulipta tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems or are on dialysis.
- have liver problems.
- are pregnant or plan to become pregnant. It is not known if Qulipta will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Qulipta passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while taking Qulipta.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Qulipta may affect the way other medicines work, and other medicines may affect how Qulipta works. Your healthcare provider may need to change the dose of Qulipta when taken with certain other medicines.
Especially tell your healthcare provider if you take any of the following, as your healthcare provider may need to change the dose of Qulipta:
- ketoconazole or itraconazole
- rifampin
- St. John’s wort
- cyclosporine
- carbamazepine
- efavirenz
- clarithromycin
- phenytoin
- etravirine
Keep a list of medicines you take to show to your healthcare provider or pharmacist when you get a new medicine.
How should I take Qulipta?
- Take Qulipta by mouth 1 time each day with or without food.
- Take Qulipta exactly as your healthcare provider tells you to take it.
What are the possible side effects of Qulipta?
The most common side effects of Qulipta include: nausea, constipation, and fatigue.
These are not all of the possible side effects of Qulipta. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Qulipta
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Qulipta for a condition for which it was not prescribed. Do not give Qulipta to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Qulipta that is written for health professionals.
How should I store Qulipta?
- Store Qulipta at room temperature between 68ºF to 77ºF (20ºC to 25ºC).
Keep Qulipta and all medicines out of the reach of children.
What are the ingredients in Qulipta?
Active ingredient: atogepant
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, mannitol, microcrystalline cellulose, polyvinylpyrrolidone vinyl acetate copolymer, sodium chloride, sodium stearyl fumarate, and vitamin E polyethylene glycol succinate.
Label
PRINCIPAL DISPLAY PANEL
- NDC 0074-7095-30
QULIPTA™
(atogepant) tablets
10 mg - Rx Only
Contains 30 Tablets
PRINCIPAL DISPLAY PANEL
- NDC 0074-7096-04
- QULIPTA™
(atogepant) tablets
Rx Only - Professional Sample – Not For Sale
- 30 mg
- Contains 4 Tablets
- For more information, visit QULIPTA.com
PRINCIPAL DISPLAY PANEL