ProAir Digihaler
Generic name: Albuterol Digital Inhalation Powder
Drug class: Adrenergic bronchodilators
Medically reviewed by A Ras MD.
What is ProAir Digihaler?
ProAir Digihaler is a prescription medicine used in people 4 years of age and older to treat or prevent bronchospasm in people who have reversible obstructive airway disease, prevent exercise-induced bronchospasm
ProAir Digihaler contains a built-in electronic module that records and stores information about inhaler events. The ProAir Digihaler may be used with, and transmits information to, an App through Bluetooth wireless technology.
ProAir Digihaler does not need to be connected to the app in order for you to take your medicine. The electronic module does not control or interfere with delivery of the medicine through the inhaler.
It is not known if ProAir Digihaler is safe and effective in children under 4 years of age.
Description
The active ingredient of ProAir Digihaler inhalation powder is albuterol sulfate, a racemic salt of albuterol. Albuterol sulfate is a beta2-adrenergic agonist. It has the chemical name α1-[(tert-butylamino) methyl]-4-hydroxy-m-xylene-α,α’-diol sulfate (2:1) (salt), and the following chemical structure:
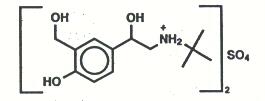
The molecular weight of albuterol sulfate is 576.7, and the empirical formula is (C13H21NO3)2•H2SO4. Albuterol sulfate is a white to off-white crystalline powder. It is soluble in water and slightly soluble in ethanol. Albuterol sulfate is the official U.S. Adopted Name in the United States, and salbutamol sulfate is the recommended World Health Organization international nonproprietary name.
ProAir Digihaler is inhalation-driven, multi-dose inhalation powder (dry powder inhaler) for oral inhalation only. It contains a formulation blend of albuterol sulfate with alpha-lactose monohydrate. Each actuation provides a metered dose of 2.6 mg of the formulation containing 117 mcg of albuterol sulfate (equivalent to 97 mcg of albuterol base) and lactose from the device reservoir. Under standardized in vitro test conditions with fixed flow rates ranging from 58 to 71 L/min, and with a total air volume of 2 L, ProAir Digihaler inhaler delivers 108 mcg of albuterol sulfate (equivalent to 90 mcg of albuterol base) with lactose from the mouthpiece. The actual amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow profile. In a study that investigated the peak inspiratory flow rate (PIFR) in asthma (n=27, ages 12 to 17 years old and n=50, ages 18 to 45 years old) and COPD (n=50, over 50 years old) patients, the mean PIFR achieved by subjects was >60 L/min (range = 31 to 110 L/min.), indicating that patients would be able to achieve the required inspiratory flow to operate the MDPI device correctly. The inhaler is provided for 200 actuations (inhalations).
ProAir Digihaler contains a QR code on the electronic module which is built-in to the top of the inhaler and automatically detects, records and stores data on inhaler events, including peak inspiratory flow rate (L/min). ProAir Digihaler may pair with and transmit data to the mobile App where inhaler events are categorized.
Mechanism of Action
Albuterol sulfate is a beta2-adrenergic agonist. The pharmacologic effects of albuterol sulfate are attributable to activation of beta2-adrenergic receptors on airway smooth muscle. Activation of beta2-adrenergic receptors leads to the activation of adenylcyclase and to an increase in the intracellular concentration of cyclic-3′,5’‑adenosine monophosphate (cyclic AMP). This increase of cyclic AMP is associated with the activation of protein kinase A, which in turn inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in muscle relaxation. Albuterol relaxes the smooth muscle of all airways, from the trachea to the terminal bronchioles. Albuterol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. Increased cyclic AMP concentrations are also associated with the inhibition of release of mediators from mast cells in the airway. While it is recognized that beta2-adrenergic receptors are the predominant receptors on bronchial smooth muscle, data indicate that there are beta-receptors in the human heart, 10% to 50% of which are cardiac beta2-adrenergic receptors. The precise function of these receptors has not been established [see Warnings and Precautions.
Albuterol has been shown in most controlled clinical trials to have more effect on the respiratory tract, in the form of bronchial smooth muscle relaxation, than isoproterenol at comparable doses while producing fewer cardiovascular effects. However, inhaled albuterol, like other beta-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes [see Warnings and Precautions
Who should not use ProAir Digihaler?
Do not use ProAir Digihaler if you are allergic to albuterol sulfate, lactose, milk proteins, or any of the ingredients in ProAir Digihaler. See the end of this guide for a complete list of ingredients in ProAir Digihaler.
What should I tell my healthcare provider before using ProAir Digihaler?
Before using ProAir Digihaler, tell your doctor about all of your medical conditions, including if you:
- have heart problems
- have high blood pressure (hypertension)
- have convulsions (seizures)
- have thyroid problems
- have diabetes
- have low potassium levels in your blood
- are pregnant or plan to become pregnant. It is not known if ProAir Digihaler will harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if ProAir Digihaler passes into your breast milk. Talk to your doctor about the best way to feed your baby if you are using ProAir Digihaler.
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
ProAir Digihaler and other medicines may affect each other and cause side effects. ProAir Digihaler may affect the way other medicines work, and other medicines may affect the way ProAir Digihaler works.
Especially tell your doctor if you take:
- other inhaled medicines or asthma medicines
- beta blocker medicines
- diuretics
- digoxin
- monoamine oxidase inhibitors
- tricyclic antidepressants
Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I use ProAir Digihaler?
- For detailed instructions, on how to use the inhaler see the “Instructions for Use” at the end of this Patient Information.
- For detailed instructions on how to set up the App go to www.ProAirDigihaler.com or call Teva at 1-888-603-0788.
- Connection to the App, having your Bluetooth turned on, or being near your smartphone is not required for your ProAir Digihaler to work and for you to get your medicine.
- The electronic module does not control or interfere with delivery of the medicine through the inhaler.
- Use ProAir Digihaler exactly as your doctor tells you to use it.
- If your child needs to use ProAir Digihaler, watch your child closely to make sure your child uses the inhaler correctly. Your doctor will show you how your child should use ProAir Digihaler.
- Each dose of ProAir Digihaler should last up to 4 hours to 6 hours.
- Do not increase your dose or take extra doses of ProAir Digihaler without first talking to your doctor.
- Do not use a spacer or volume holding chamber with ProAir Digihaler.
- ProAir Digihaler does not need priming.
- Get medical help right away if ProAir Digihaler no longer helps your symptoms.
- Get medical help right away if your symptoms get worse or if you need to use your inhaler more often.
- While you are using ProAir Digihaler, do not use other inhaled rescue medicines and asthma medicines unless your doctor tells you to do so.
Call your doctor if your asthma symptoms, like wheezing and trouble breathing, become worse over a few hours or days. Your doctor may need to give you another medicine (for example, corticosteroids) to treat your symptoms.
What are the possible side effects of ProAir Digihaler?
ProAir Digihaler may cause serious side effects, including:
- worsening trouble breathing, coughing and wheezing (paradoxical bronchospasm). If this happens stop using ProAir Digihaler and call your doctor or get emergency help right away. Paradoxical bronchospasm is more likely to happen with your first use of a new asthma inhalation medicine.
- heart problems, including faster heart rate and higher blood pressure
- possible death in people with asthma who use too much ProAir Digihaler
- allergic reactions. Call your doctor right away if you have the following symptoms of an allergic reaction:
- itchy skin
- swelling beneath your skin or in your throat
- rash
- worsening trouble breathing
- worsening of other medical problems in people who also use ProAir Digihaler including increases in blood sugar
- low potassium levels in your blood
The most common side effects of ProAir Digihaler include:
- back pain
- pain
- upset stomach
- sinus headache
- urinary tract infection
- your heart feels like it is pounding or racing (palpitations)
- chest pain
- fast heart rate
- shakiness
- nervousness
- headache
- dizziness
- sore throat
- runny nose
These are not all of the possible side effects of ProAir Digihaler.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of ProAir Digihaler
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ProAir Digihaler for a condition for which it was not prescribed. Do not give ProAir Digihaler to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or doctor for information about ProAir Digihaler that was written for health professionals.
How should I store ProAir Digihaler?
- Store ProAir Digihaler at room temperature between 59ºF and 77ºF (15ºC and 25ºC).
- Avoid exposure to extreme heat, cold, or humidity.
- Keep the cap on the inhaler closed during storage.
- Keep your ProAir Digihaler inhaler dry and clean at all times.
- Do not wash or put any part of your ProAir Digihaler inhaler in water. Replace your inhaler if washed or placed in water.
Keep ProAir Digihaler and all medicines out of the reach of children.
What are the ingredients in ProAir Digihaler?
Active ingredient: albuterol sulfate
Inactive ingredients: lactose (may contain milk proteins)
For more information about ProAir Digihaler, call1-888-603-0788.or go to www.ProAirDigihaler.com
Instructions for use for ProAir Digihaler
ProAir Digihaler (prō´ār di´ji haye´´ler)
(albuterol sulfate)
Inhalation powder
Your ProAir Digihaler Inhaler
When you are ready to use ProAir Digihaler for the first time, remove the ProAir Digihaler inhaler from the foil pouch.
There are 3 main parts of your ProAir Digihaler inhaler including:
- the white inhaler with the mouthpiece. See Figure A.
- the red cap that covers the mouthpiece and vent of the inhaler. See Figure A.
- the electronic module. See Figure A.
There is an electronic module built into the top of the inhaler that records and stores information about inhaler events. The electronic module sends information through Bluetooth® wireless technology to a mobile application (App). The electronic module does not control or interfere with delivery of the medicine through the inhaler.There is a dose counter in the back of the inhaler with a viewing window that shows you how many doses of medicine you have left. See Figure A.
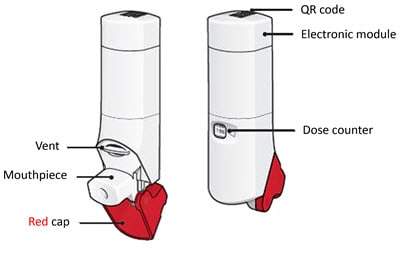
Figure A
- Your ProAir Digihaler inhaler contains 200 doses (inhalations). See Figure B.
- The dose counter shows the number of doses left in your inhaler.
- When there are 20 doses left, the dose counter will change to red and you should refill your prescription or ask your doctor for another prescription.
- When the dose counter displays ‘0’ your inhaler is empty and you should stop using the inhaler and throw it away. See Figure B.
 Figure B
Figure B
IMPORTANT:
- Always close the cap after each inhalation so your inhaler will be ready for you to take your next dose. Do not open the cap unless you are ready for your next dose.
- You will hear a “click” sound when the cap is opened fully. If you do not hear the “click” sound the inhaler may not be activated to give you a dose of medicine.
- ProAir Digihaler does not have an activation button or medicine canister. When you open the cap, a dose of ProAir Digihaler will be activated for delivery of the medicine.
- ProAir Digihaler does not need to be wirelessly connected to the mobile application (App) in order for it to work and for you to take your medicine.
- In general, the technique for administering ProAir Digihaler to children is similar to that for adults. Children should use ProAir Digihaler under adult supervision, as instructed by the patient’s physician.
- Do not use a spacer or volume holding chamber with ProAir Digihaler. ProAir Digihaler does not need priming.
Using your ProAir Digihaler inhaler:
Important: Make sure the red cap is closed before you start using your inhaler.
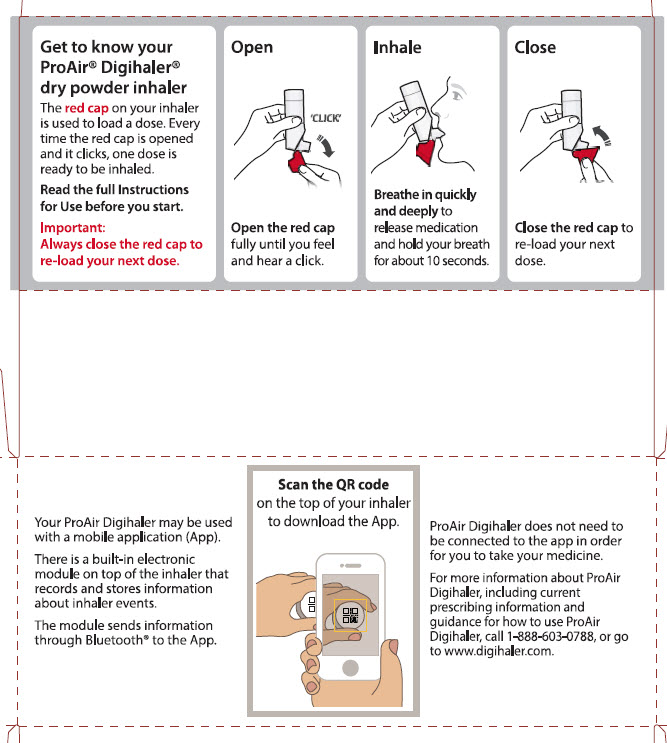
Step 1. Open
- Hold the inhaler upright and open the red cap fully until you feel and hear a “click”. See Figure C.
- Each time you open the red cap and it “clicks”, a dose of ProAir Digihaler is ready to be inhaled.
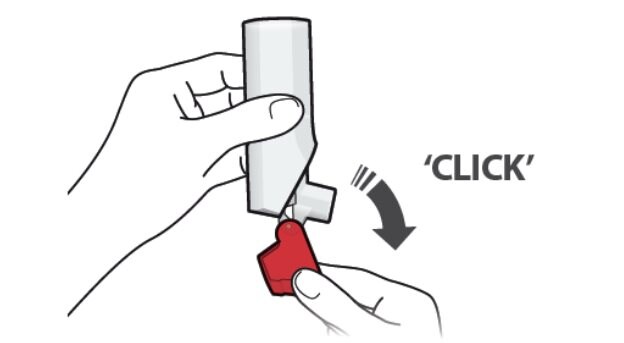
Figure C
Remember:
- For the correct use of ProAir Digihaler, hold the inhaler upright as you open the red cap. See Figure D.
- Do not hold the inhaler in any other way as you open the red cap.
- Do not open the red cap until you are ready to take a dose of ProAir Digihaler.
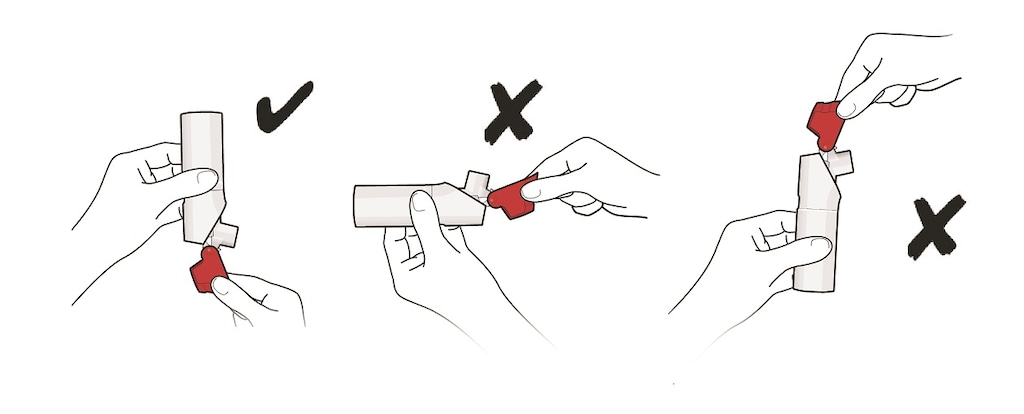 Figure D
Figure D
Step 2. Inhale
- Before you inhale, breathe out (exhale) through your mouth and push as much air from your lungs as you can. See Figure E.
- Do not exhale into the inhaler mouthpiece.
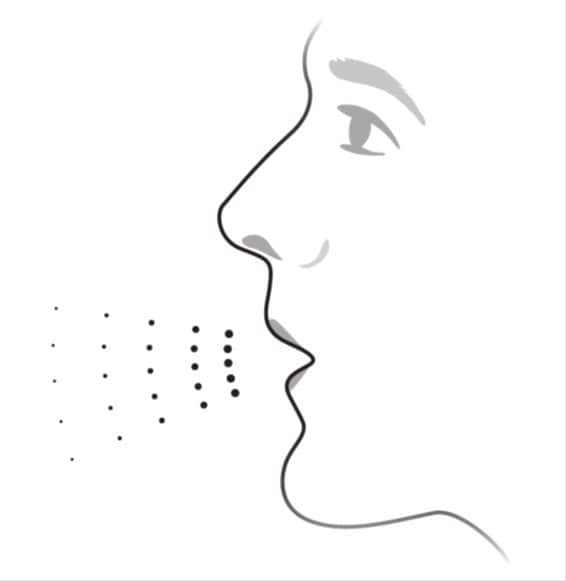
Figure E
- Put the mouthpiece in your mouth and close your lips tightly around it. See Figure F.
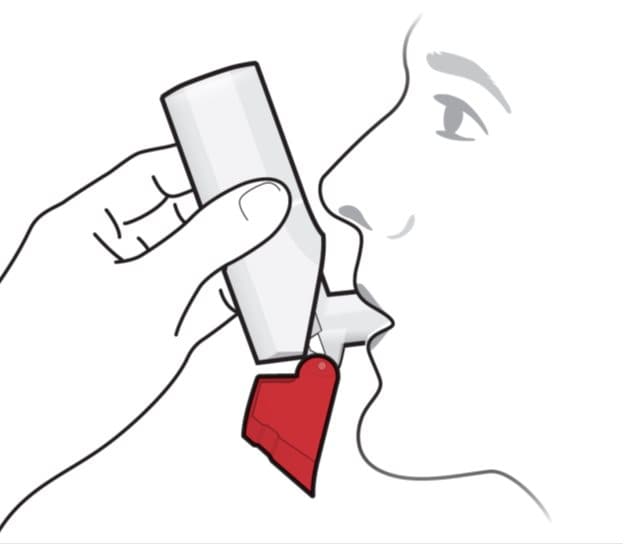
Figure F
- Do not block the vent above the mouthpiece with your lips or fingers. See Figure G.
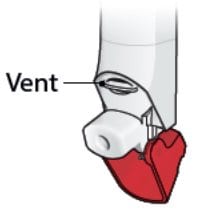
Figure G
- Breathe in quickly and deeply through your mouth, to deliver the dose of medicine to your lungs.
- Remove the inhaler from your mouth.
- Hold your breath for about 10 seconds or for as long as you comfortably can.
- Your ProAir Digihaler Inhaler delivers your dose of medicine as a very fine powder that you may or may not taste or feel. Do not take an extra dose from the inhaler even if you do not taste or feel the medicine.
Step 3. Close
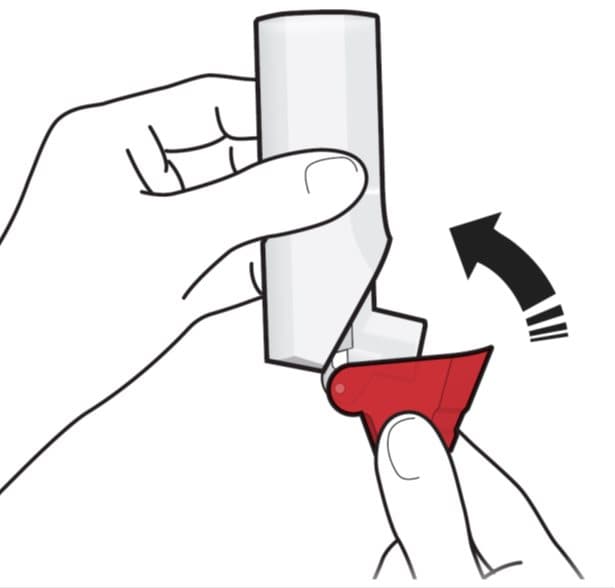
Figure H
- Close the red cap firmly over the mouthpiece. See Figure H.
- Make sure you close the red cap after each inhalation so that the inhaler will be ready for your next dose.
- If you need another dose, close the red cap and then repeat steps 1-3.
Storing your ProAir Digihaler inhaler
- Store ProAir Digihaler at room temperature between 59ºF and 77ºF (15ºC and 25ºC).
- Avoid exposure to extreme heat, cold, or humidity.
- Keep the red cap on the inhaler closed during storage.
- Keep your ProAir Digihaler inhaler dry and clean at all times.
- Do not wash or put any part of your ProAir Digihaler inhaler in water. Replace your inhaler if washed or placed in water.
- Keep your ProAir Digihaler inhaler and all medicines out of the reach of children.
Cleaning your ProAir Digihaler inhaler
- Do not wash or put any part of your ProAir Digihaler inhaler in water. Replace your inhaler if washed or placed in water.
- ProAir Digihaler contains a powder and must be kept clean and dry at all times.
- If the mouthpiece needs cleaning, gently wipe it with a dry cloth or tissue.
Replacing your ProAir Digihaler inhaler
- The dose counter on the back of your inhaler shows how many doses you have left. Do not try to change the numbers for the dose counter.
- When there are 20 doses left, the dose counter color will change to red and you should refill your prescription or ask your doctor for another prescription.
- When the dose counter displays ‘0’ your ProAir Digihaler inhaler is empty and you should stop using the inhaler and throw it away.
- Throw away your ProAir Digihaler inhaler 13 months after removing it from the foil pouch for the first time, when the dose counter displays ‘0’, or after the expiration date on the package, whichever comes first.
- ProAir Digihaler contains a lithium – manganese dioxide battery and should be thrown away (disposed of) in accordance with state and local regulations.
Important information
- Do not open the red cap unless you are taking a dose. Repeatedly opening and closing the cap without inhaling a dose will waste the medicine and may damage your inhaler.
- Your ProAir Digihaler inhaler contains dry powder so it is important that you do not blow or breathe into it.
- Do not take the inhaler apart.
Support
- For instructions on setting up the App, go to www.ProAirDigihaler.com or call Teva at 1-888-603-0788.
- If you have any questions about ProAir Digihaler, how to use your inhaler, go to WWW.PROAIRDIGIHALER.COM or call 1-888-603-0788.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1) This device may not cause harmful interference, and
(2) This device must accept any interference received, including interference that may cause undesired operation. Changes or modifications not expressly approved by Teva could void the user’s authority to operate the equipment.
Label
PACKAGING/LABEL DISPLAY PANEL 59310-117-20
- NDC 59310-117-20
Rx Only - ProAir digihaler (albuterol sulfate) Inhalation Powder
- 200 metered inhalations
- 0.65g net contents
- Refer to enclosed Patient Leaflet and Instructions for Use for detailed information on product use and handling.
- For more information visit www.ProAirDigihaler.com or call 1-800-603-0788

PACKAGING/LABEL DISPLAY PANEL 59310-540-20
- NDC 59310-540-20
Rx Only - ProAir digihaler (albuterol sulfate) Inhalation Powder
- SAMPLE NOT FOR SALE
- 200 metered inhalations
- 0.65g net contents
- Refer to enclosed Patient Leaflet and Instructions for Use for detailed information on product use and handling.
- For more information visit www.ProAirDigihaler.com or call 1-800-603-0788
SRC: NLM .
