Prevymis
Generic name: letermovir (oral/injection)
Drug class: Miscellaneous antivirals
Medically reviewed by A Ras MD.
What is Prevymis?
Prevymis is a prescription medicine to help to prevent cytomegalovirus (CMV) infection and disease in adults who have received an allogeneic hematopoietic stem cell (bone marrow) transplant.
It is not known if Prevymis is safe and effective in children under 18 years of age.
Description
PREVYMIS contains letermovir, an inhibitor of the CMV DNA terminase complex, and is administered orally or by intravenous infusion.
PREVYMIS is available as 240 mg and 480 mg tablets. PREVYMIS tablets contain either 240 mg or 480 mg of letermovir and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, povidone 25, and film-coated with a coating material containing the following inactive ingredients: hypromellose 2910, iron oxide red (only for 480 mg tablets), iron oxide yellow, lactose monohydrate, titanium dioxide, and triacetin. Carnauba wax is added as a polishing agent.
PREVYMIS is also available as 240 mg and 480 mg injection for intravenous infusion. PREVYMIS injection is a clear, preservative-free sterile solution and may contain a few small translucent or white particles in single-dose vials of either 240 mg or 480 mg per vial. Each 1 mL of solution contains 20 mg letermovir, hydroxypropyl betadex (150 mg), sodium chloride (3.1 mg), sodium hydroxide (1.2 mg), and Water for Injection, USP. The amount of sodium hydroxide may be adjusted to achieve a pH of approximately 7.5.
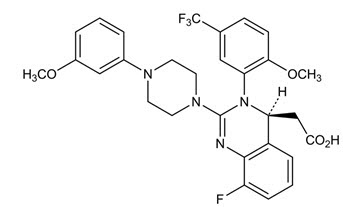
Letermovir has a molecular formula of C29H28F4N4O4 and a molecular weight of 572.55. The chemical name for letermovir is (4S)-2-{8-Fluoro-2-[4-(3- methoxyphenyl)piperazin-1-yl]-3-[2-methoxy-5- (trifluoromethyl)phenyl]-3,4-dihydroquinazolin-4-yl}acetic acid. Letermovir is very slightly soluble in water.
The chemical structure of letermovir is:
Who should not take Prevymis?
Do not take Prevymis if you take:
- Pimozide
- Ergot alkaloids
If you are taking Prevymis with cyclosporine, do not take:
- Pitavastatin or simvastatin
What should I tell my healthcare provider before taking Prevymis?
Tell your doctor about all your medical conditions, including if you:
- Have kidney or liver problems.
- Are pregnant or plan to become pregnant. It is not known if Prevymis will harm your unborn baby.
- Are breastfeeding or plan to breastfeed. It is not known if Prevymis passes into your breast milk. Talk to your doctor about the best way to feed your baby while taking Prevymis.
Tell your doctor about all of the medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements. Prevymis may affect the way other medicines work, and other medicines may affect how Prevymis works and cause serious side effects.
Know the medicines you take. Keep a list of medicines and show it to your doctor and pharmacist when you get a new medicine. Your doctor or pharmacist will tell you if it is safe to take Prevymis with other medicines. Do not start or stop taking another medicine without telling your doctor first.
How should I take Prevymis?
Prevymis comes as a tablet or can be given by your doctor through an IV line (intravenously).
- If you take Prevymis tablets:
- Take Prevymis exactly as your doctor tells you to take it. Do not stop taking Prevymis without talking to your doctor first.
- Take 1 Prevymis tablet once a day.
- Take Prevymis with or without food.
- Swallow Prevymis tablets whole.
- It is important that you do not miss or skip doses of Prevymis.
- If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and take your dose at the next scheduled time. Do not take 2 doses of Prevymis at the same time to make up for a missed dose.
- If you take too much Prevymis, call your doctor right away.
- If you receive Prevymis through an IV line (intravenously):
- You will receive Prevymis 1 time each day given over 1 hour.
- If you miss or skip your dose of Prevymis, call your doctor right away.
What are the possible side effects of Prevymis?
The most common side effects while taking Prevymis include:
- nausea
- diarrhea
- vomiting
- swelling in your arms and legs
- cough
- headache
- tiredness
- stomach (abdominal) pain
These are not all the possible side effects of Prevymis.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of Prevymis
Medicines are sometimes prescribed for purposes other than those listed in the Patient Information leaflet. Do not use Prevymis for a condition for which it was not prescribed. Do not give Prevymis to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Prevymis that is written for healthcare professionals.
How should I store Prevymis?
- Store Prevymis tablets and Prevymis injection at room temperature between 68°F to 77°F (20°C to 25°C).
- Store Prevymis tablets in the original package until you are ready to take it.
- Store Prevymis injection in the original carton to protect from exposure to light.
Keep Prevymis and all medicines out of the reach of children.
What are the ingredients in Prevymis?
Active ingredient: letermovir
Inactive ingredients:
Tablets: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and povidone 25. Film coating: hypromellose 2910, iron oxide red (only for 480 mg tablets), iron oxide yellow, lactose monohydrate, titanium dioxide, triacetin. Carnauba wax is added as a polishing agent.
Injection: hydroxypropyl betadex, sodium chloride, sodium hydroxide, and Water for Injection, USP.
Label
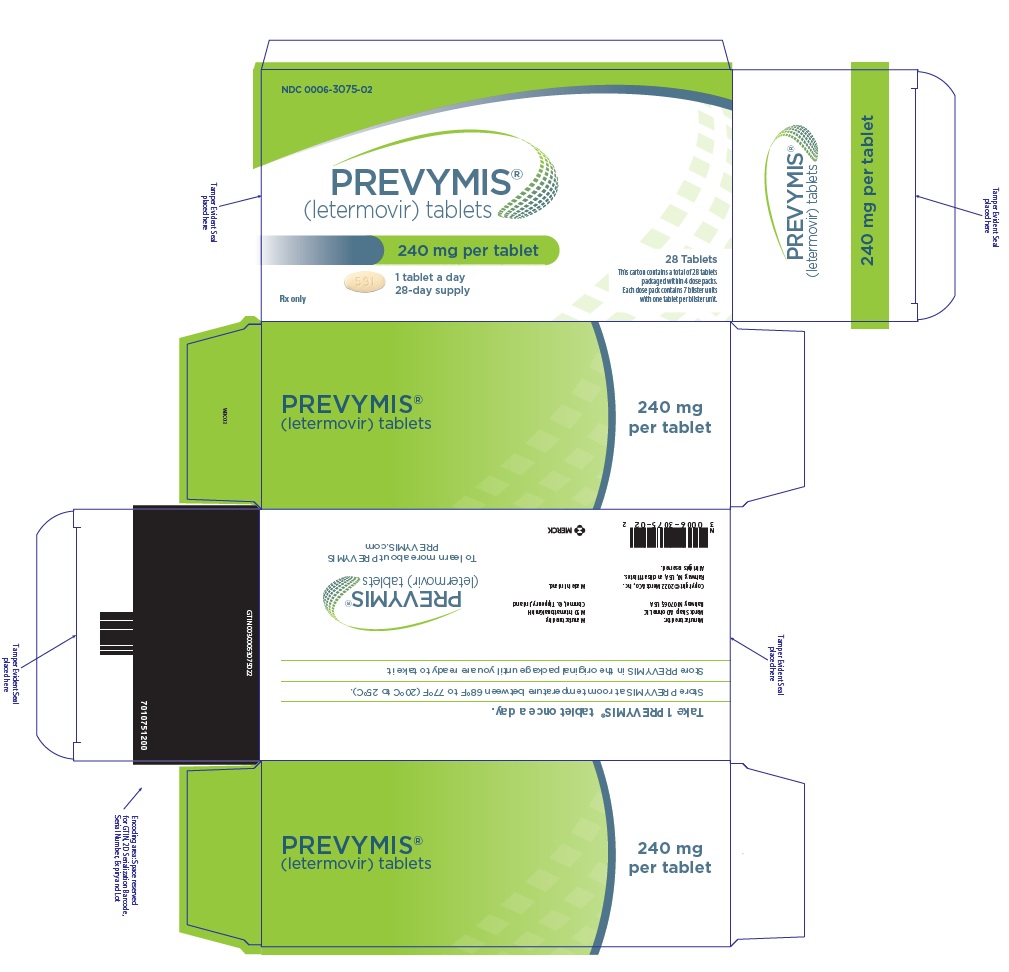
PRINCIPAL DISPLAY PANEL – 240 MG TABLET DOSE PACK CARTON
- NDC 0006-3075-02
- PREVYMIS™
(letermovir) tablets - 240 mg per tablet
- 1 tablet a day
28-day supply - Rx only
- 28 Tablets
- This carton contains a total of 28 tablets
packaged within 4 dose packs.
Each dose pack contains 7 blister units
with one tablet per blister unit.
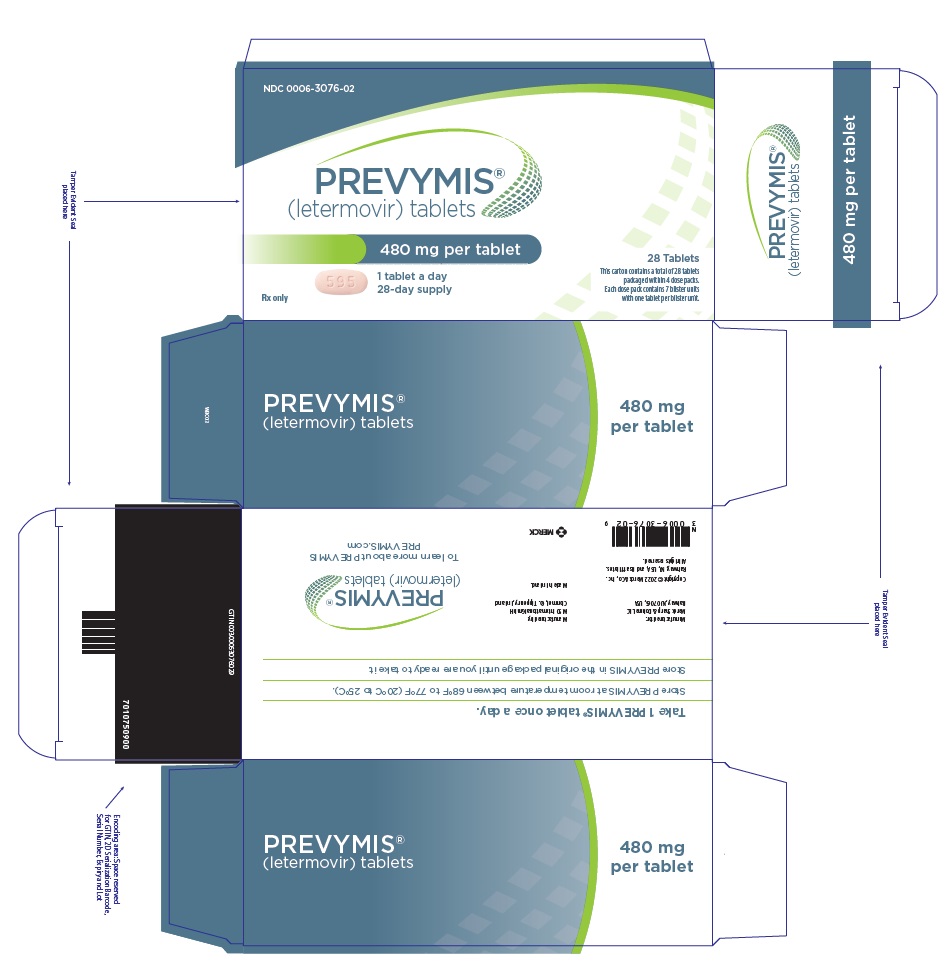
PRINCIPAL DISPLAY PANEL – 480 MG TABLET DOSE PACK CARTON
- NDC 0006-3076-02
- PREVYMIS™
(letermovir) tablets - 480 mg per tablet
- 1 tablet a day
28-day supply - Rx only
- 28 Tablets
- This carton contains a total of 28 tablets
packaged within 4 dose packs.
Each dose pack contains 7 blister units
with one tablet per blister unit.

SRC: NLM .